Neferine inhibits BMECs pyroptosis and maintains blood-brain barrier integrity in ischemic stroke by triggering a cascade reaction of PGC-1α
- PMID: 38910141
- PMCID: PMC11194274
- DOI: 10.1038/s41598-024-64815-w
Neferine inhibits BMECs pyroptosis and maintains blood-brain barrier integrity in ischemic stroke by triggering a cascade reaction of PGC-1α
Abstract
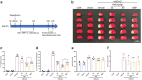
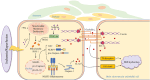
Blood-brain barrier disruption is a critical pathological event in the progression of ischemic stroke (IS). Most studies regarding the therapeutic potential of neferine (Nef) on IS have focused on neuroprotective effect. However, whether Nef attenuates BBB disruption during IS is unclear. We here used mice underwent transient middle cerebral artery occlusion (tMCAO) in vivo and bEnd.3 cells exposed to oxygen-glucose deprivation/reoxygenation (OGD/R) injury in vitro to simulate cerebral ischemia. We showed that Nef reduced neurobehavioral dysfunction and protected brain microvascular endothelial cells and BBB integrity. Molecular docking, short interfering (Si) RNA and plasmid transfection results showed us that PGC-1α was the most binding affinity of biological activity protein for Nef. And verification experiments were showed that Nef upregulated PGC-1α expression to reduce mitochondrial oxidative stress and promote TJ proteins expression, further improves the integrity of BBB in mice. Intriguingly, our study showed that neferine is a natural PGC-1α activator and illustrated the mechanism of specific binding site. Furthermore, we have demonstrated Nef reduced mitochondria oxidative damage and ameliorates endothelial inflammation by inhibiting pyroptosis to improve BBB permeability through triggering a cascade reaction of PGC-1α via regulation of PGC-1α/NLRP3/GSDMD signaling pathway to maintain the integrity of BBB in ischemia/reperfusion injury.
Keywords: Blood–Brain Barrier; Ischemic stroke; Neferine; Oxygen and glucose-deprivation/reperfusion; PGC-1α; Transient middle cerebral artery occlusion.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Chen S-D, et al. Activation of calcium/calmodulin-dependent protein kinase IV and peroxisome proliferator-activated receptor γ coactivator-1α signaling pathway protects against neuronal injury and promotes mitochondrial biogenesis in the hippocampal CA1 subfield after transient global ischemia. J. Neurosci. Res. 2010 doi: 10.1002/jnr.22469. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

