Increasing correlation between oral and gastric microbiota during gastric carcinogenesis
- PMID: 38910513
- PMCID: PMC11236816
- DOI: 10.3904/kjim.2023.490
Increasing correlation between oral and gastric microbiota during gastric carcinogenesis
Abstract
Background/aims: Recent research has increasingly focused on the role of the gastric microbiome in the development of gastric cancer. We aimed to investigate the changes in the microbiome during gastric carcinogenesis in structural and functional aspects, with a specific focus on the association between oral and gastric microbiomes.
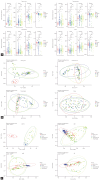
Methods: We collected saliva, gastric juice, and gastric tissue samples from 141 patients at different stages of gastric carcinogenesis and processed them for microbiome analysis using 16S rRNA gene profiling. The alpha and beta diversities were analyzed, and the differences in microbiome composition and function profiles were analyzed among the groups, as well as the correlation between changes in the oral and gastric microbiomes during carcinogenesis.
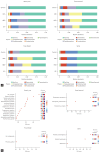
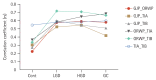
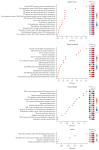
Results: We observed significant differences in microbial diversity and composition between the disease and control groups, primarily in the gastric juice. Specific bacterial strains, including Schaalia odontolytica, Streptococcus cristatus, and Peptostreptococcus stomatis, showed a significant increase in abundance in the gastric juice in the low-grade dysplasia and gastric cancer groups. Notably, the correlation between the oral and gastric microbiota compositions, increased as the disease progressed. Predictive analysis of the metagenomic functional profiles revealed changes in functional pathways that may be associated with carcinogenesis (ABC transport and two-component systems).
Conclusion: During gastric carcinogenesis, the abundance of oral commensals associated with cancer increased in the stomach. The similarity in microbial composition between the stomach and oral cavity also increased, implying a potential role of oral-gastric bacterial interactions in gastric cancer development.
Keywords: 16S rRNA; Carcinogenesis; Gastric cancer; Microbiome.
Conflict of interest statement
The authors disclose no conflicts.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54(7 Suppl):1941s–1943s. - PubMed
-
- Eun CS, Kim BK, Han DS, et al. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter. 2014;19:407–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
