Impaired Intracellular Calcium Buffering Contributes to the Arrhythmogenic Substrate in Atrial Myocytes From Patients With Atrial Fibrillation
- PMID: 38910563
- PMCID: PMC11319087
- DOI: 10.1161/CIRCULATIONAHA.123.066577
Impaired Intracellular Calcium Buffering Contributes to the Arrhythmogenic Substrate in Atrial Myocytes From Patients With Atrial Fibrillation
Abstract
Background: Alterations in the buffering of intracellular Ca2+, for which myofilament proteins play a key role, have been shown to promote cardiac arrhythmia. It is interesting that although studies report atrial myofibrillar degradation in patients with persistent atrial fibrillation (persAF), the intracellular Ca2+ buffering profile in persAF remains obscure. Therefore, we aimed to investigate the intracellular buffering of Ca2+ and its potential arrhythmogenic role in persAF.
Methods: Transmembrane Ca2+ fluxes (patch-clamp) and intracellular Ca2+ signaling (fluo-3-acetoxymethyl ester) were recorded simultaneously in myocytes from right atrial biopsies of sinus rhythm (Ctrl) and patients with persAF, alongside human atrial subtype induced pluripotent stem cell-derived cardiac myocytes (iPSC-CMs). Protein levels were quantified by immunoblotting of human atrial tissue and induced pluripotent stem cell-derived cardiac myocytes. Mouse whole heart and atrial electrophysiology were measured on a Langendorff system.
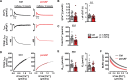
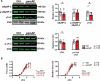
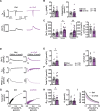
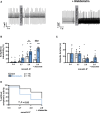
Results: Cytosolic Ca2+ buffering was decreased in atrial myocytes of patients with persAF because of a depleted amount of Ca2+ buffers. In agreement, protein levels of selected Ca2+ binding myofilament proteins, including cTnC (cardiac troponin C), a major cytosolic Ca2+ buffer, were significantly lower in patients with persAF. Small interfering RNA (siRNA)-mediated knockdown of cTnC (si-cTNC) in atrial iPSC-CM phenocopied the reduced cytosolic Ca2+ buffering observed in persAF. Si-cTnC treated atrial iPSC-CM exhibited a higher predisposition to spontaneous Ca2+ release events and developed action potential alternans at low stimulation frequencies. Last, indirect reduction of cytosolic Ca2+ buffering using blebbistatin in an ex vivo mouse whole heart model increased vulnerability to tachypacing-induced atrial arrhythmia, validating the direct mechanistic link between impaired cytosolic Ca2+ buffering and atrial arrhythmogenesis.
Conclusions: Our findings suggest that loss of myofilament proteins, particularly reduced cTnC protein levels, causes diminished cytosolic Ca2+ buffering in persAF, thereby potentiating the occurrence of spontaneous Ca2+ release events and atrial fibrillation susceptibility. Strategies targeting intracellular buffering may represent a promising therapeutic lead in persAF management.
Keywords: atrial fibrillation; atrial remodeling; calcium signaling; cardiac arrhythmias; electrophysiology; ion channels.
Conflict of interest statement
None.
Figures




References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad - PMC - PubMed
-
- Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, et al. . EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028 - PMC - PubMed
-
- Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, et al. . Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306 - PMC - PubMed
-
- Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol. 2008;52:293–299. doi: 10.1097/FJC.0b013e318171924d - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

