Targeting pyruvate kinase M2 for the treatment of kidney disease
- PMID: 38910890
- PMCID: PMC11190346
- DOI: 10.3389/fphar.2024.1376252
Targeting pyruvate kinase M2 for the treatment of kidney disease
Abstract
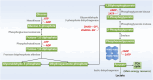
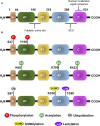
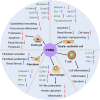
Pyruvate kinase M2 (PKM2), a rate limiting enzyme in glycolysis, is a cellular regulator that has received extensive attention and regards as a metabolic regulator of cellular metabolism and energy. Kidney is a highly metabolically active organ, and glycolysis is the important energy resource for kidney. The accumulated evidences indicates that the enzymatic activity of PKM2 is disturbed in kidney disease progression and treatment, especially diabetic kidney disease and acute kidney injury. Modulating PKM2 post-translational modification determines its enzymatic activity and nuclear translocation that serves as an important interventional approach to regulate PKM2. Emerging evidences show that PKM2 and its post-translational modification participate in kidney disease progression and treatment through modulating metabolism regulation, podocyte injury, fibroblast activation and proliferation, macrophage polarization, and T cell regulation. Interestingly, PKM2 activators (TEPP-46, DASA-58, mitapivat, and TP-1454) and PKM2 inhibitors (shikonin, alkannin, compound 3k and compound 3h) have exhibited potential therapeutic property in kidney disease, which indicates the pleiotropic effects of PKM2 in kidney. In the future, the deep investigation of PKM2 pleiotropic effects in kidney is urgently needed to determine the therapeutic effect of PKM2 activator/inhibitor to benefit patients. The information in this review highlights that PKM2 functions as a potential biomarker and therapeutic target for kidney diseases.
Keywords: acute kidney injury; diabetic kidney disease; glycolysis; post-translational modification; pyruvate kinase M2.
Copyright © 2024 Chen, Han, Liu, Feng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

