Intestinal Epithelial Axin1 Deficiency Protects Against Colitis via Altered Gut Microbiota
- PMID: 38911180
- PMCID: PMC11192507
- DOI: 10.1016/j.eng.2023.06.007
Intestinal Epithelial Axin1 Deficiency Protects Against Colitis via Altered Gut Microbiota
Abstract
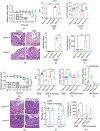
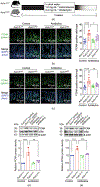
Intestinal homeostasis is maintained by specialized host cells and the gut microbiota. Wnt/β-catenin signaling is essential for gastrointestinal development and homeostasis, and its dysregulation has been implicated in inflammation and colorectal cancer. Axin1 negatively regulates activated Wnt/β-catenin signaling, but little is known regarding its role in regulating host-microbial interactions in health and disease. Here, we aim to demonstrate that intestinal Axin1 determines gut homeostasis and host response to inflammation. Axin1 expression was analyzed in human inflammatory bowel disease datasets. To explore the effects and mechanism of intestinal Axin1 in regulating intestinal homeostasis and colitis, we generated new mouse models with Axin1 conditional knockout in intestinal epithelial cell (IEC; Axin1 ΔIEC) and Paneth cell (PC; Axin1 ΔPC) to compare with control (Axin1 LoxP; LoxP: locus of X-over, P1) mice. We found increased Axin1 expression in the colonic epithelium of human inflammatory bowel disease (IBD). Axin1 ΔIEC mice exhibited altered goblet cell spatial distribution, PC morphology, reduced lysozyme expression, and enriched Akkermansia muciniphila (A. muciniphila). The absence of intestinal epithelial and PC Axin1 decreased susceptibility to dextran sulfate sodium (DSS)-induced colitis in vivo. Axin1 ΔIEC and Axin1 ΔPC mice became more susceptible to DSS-colitis after cohousing with control mice. Treatment with A. muciniphila reduced DSS-colitis severity. Antibiotic treatment did not change the IEC proliferation in the Axin1 Loxp mice. However, the intestinal proliferative cells in Axin1 ΔIEC mice with antibiotic treatment were reduced compared with those in Axin1 ΔIEC mice without treatment. These data suggest non-colitogenic effects driven by the gut microbiome. In conclusion, we found that the loss of intestinal Axin1 protects against colitis, likely driven by epithelial Axin1 and Axin1-associated A. muciniphila. Our study demonstrates a novel role of Axin1 in mediating intestinal homeostasis and the microbiota. Further mechanistic studies using specific Axin1 mutations elucidating how Axin1 modulates the microbiome and host inflammatory response will provide new therapeutic strategies for human IBD.
Keywords: Akkermansia muciniphila; Axin1; Bacteria; Immunity; Inflammatory bowel disease; Microbiome; Microbiome inflammation; Paneth cells; Wnt.
Conflict of interest statement
Compliance with ethics guidelines Shari declare Garrett, Yongguo Zhang, Yinglin Xia, and Jun Sun that they have no conflict of interest.
Figures
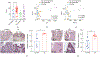







References
-
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et al. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5(1):17–30. - PMC - PubMed
-
- Moparthi L, Koch S. Wnt signaling in intestinal inflammation. Differentiation 2019;108:24–32. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
