Mussel inspired 3D elastomer enabled rapid calvarial bone regeneration through recruiting more osteoprogenitors from the dura mater
- PMID: 38911700
- PMCID: PMC11193312
- DOI: 10.1093/rb/rbae059
Mussel inspired 3D elastomer enabled rapid calvarial bone regeneration through recruiting more osteoprogenitors from the dura mater
Abstract
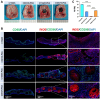
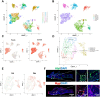
Currently, the successful healing of critical-sized calvarial bone defects remains a considerable challenge. The immune response plays a key role in regulating bone regeneration after material grafting. Previous studies mainly focused on the relationship between macrophages and bone marrow mesenchymal stem cells (BMSCs), while dural cells were recently found to play a vital role in the calvarial bone healing. In this study, a series of 3D elastomers with different proportions of polycaprolactone (PCL) and poly(glycerol sebacate) (PGS) were fabricated, which were further supplemented with polydopamine (PDA) coating. The physicochemical properties of the PCL/PGS and PCL/PGS/PDA grafts were measured, and then they were implanted as filling materials for 8 mm calvarial bone defects. The results showed that a matched and effective PDA interface formed on a well-proportioned elastomer, which effectively modulated the polarization of M2 macrophages and promoted the recruitment of dural cells to achieve full-thickness bone repair through both intramembranous and endochondral ossification. Single-cell RNA sequencing analysis revealed the predominance of dural cells during bone healing and their close relationship with macrophages. The findings illustrated that the crosstalk between dural cells and macrophages determined the vertical full-thickness bone repair for the first time, which may be the new target for designing bone grafts for calvarial bone healing.
Keywords: 3D elastomer; bone healing; dural cells; macrophages; polydopamine.
© The Author(s) 2024. Published by Oxford University Press.
Figures








Similar articles
-
Mussel patterned with 4D biodegrading elastomer durably recruits regenerative macrophages to promote regeneration of craniofacial bone.Biomaterials. 2021 Sep;276:120998. doi: 10.1016/j.biomaterials.2021.120998. Epub 2021 Jun 29. Biomaterials. 2021. PMID: 34237507
-
Poly (glycerol sebacate) elastomer supports bone regeneration by its mechanical properties being closer to osteoid tissue rather than to mature bone.Acta Biomater. 2017 May;54:95-106. doi: 10.1016/j.actbio.2017.01.053. Epub 2017 Jan 19. Acta Biomater. 2017. PMID: 28110067
-
Evaluation of new bone formation in critical-sized rat calvarial defect using 3D printed polycaprolactone/tragacanth gum-bioactive glass composite scaffolds.Int J Biol Macromol. 2024 Jun;270(Pt 1):132361. doi: 10.1016/j.ijbiomac.2024.132361. Epub 2024 May 17. Int J Biol Macromol. 2024. PMID: 38750857
-
Calvarial Suture-Derived Stem Cells and Their Contribution to Cranial Bone Repair.Front Physiol. 2017 Nov 27;8:956. doi: 10.3389/fphys.2017.00956. eCollection 2017. Front Physiol. 2017. PMID: 29230181 Free PMC article. Review.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
References
-
- Kaplani K, Koutsi S, Armenis V, Skondra FG, Karantzelis N, Champeris Tsaniras S, Taraviras S.. Wound healing related agents: ongoing research and perspectives. Adv Drug Deliv Rev 2018;129:242–53. - PubMed
-
- Hasani-Sadrabadi MM, Sarrion P, Pouraghaei S, Chau Y, Ansari S, Li S, Aghaloo T, Moshaverinia A.. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med 2020;12:eaay6853. - PubMed
LinkOut - more resources
Full Text Sources

