Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review
- PMID: 38911761
- PMCID: PMC11190924
- DOI: 10.1021/acsomega.4c00933
Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review
Abstract
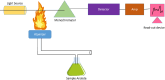
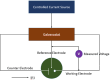
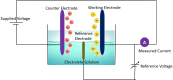
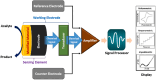
Heavy metal ions (HMIs) are very harmful to the ecosystem when they are present in excess of the recommended limits. They are carcinogenic in nature and can cause serious health issues. So, it is important to detect the metal ions quickly and accurately. The metal ions arsenic (As3+), cadmium (Cd2+), chromium (Cr3+), lead (Pb2+), and mercury (Hg2+) are considered to be very toxic among other metal ions. Standard analytical methods like atomic absorption spectroscopy, atomic fluorescence spectroscopy, and X-ray fluorescence spectroscopy are used to detect HMIs. But these methods necessitate highly technical equipment and lengthy procedures with skilled personnel. So, electrochemical sensing methods are considered to be more advantageous because of their quick analysis with precision and simplicity to operate. They can detect a wide range of heavy metals providing real-time monitoring and are cost-effective and enable multiparametric detection. Various sensing applications necessitate severe regulation regarding the modification of electrode surfaces. Numerous nanomaterials such as graphene, carbon nanotubes, and metal nanoparticles have been extensively explored as interface materials in electrode modifiers. These nanoparticles offer excellent electrical conductivity, distinctive catalytic properties, and high surface area resulting in enhanced electrochemical performance. This review examines different HMI detection methods in an aqueous medium by an electrochemical sensing approach and studies the recent developments in interface materials for altering the electrodes.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Duffus J. H. Heavy metal–a meaningless term. Pure Appl. Chem. 2002, 74 (5), 793–807. 10.1351/pac200274050793. - DOI
-
- Musarrat J.; Zaidi A.; Khan M. S.; Siddiqui M. A.; Al-Khedhairy A. A.. Genotoxicity Assessment of Heavy Metal–Contaminated Soils. In Bio management of Metal-Contaminated Soils. Environmental Pollution; Khan M., Zaidi A., Goel R., Musarrat J., Eds.; Springer: 2011; Vol. 20. 10.1007/978-94-007-1914-9_14. - DOI
-
- Prabhakar S.; Singh A. K.; Pooni D. S. Effect of environmental pollution on animal and human health: a review. Indian Journal of Animal Sciences. 2012, 82 (3), 244.
-
- Martin S.; Griswold W. Human health effects of heavy metals. Environmental Science and Technology briefs for citizens. 2009, 15, 1–6.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
