Removal of Carbon Dioxide and Hydrogen Sulfide from Natural Gas Using a Hybrid Solvent of Monoethanolamine and N-Methyl 2-Pyrrolidone
- PMID: 38911790
- PMCID: PMC11191085
- DOI: 10.1021/acsomega.3c09100
Removal of Carbon Dioxide and Hydrogen Sulfide from Natural Gas Using a Hybrid Solvent of Monoethanolamine and N-Methyl 2-Pyrrolidone
Abstract
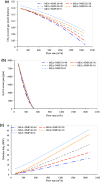
The main goal of traditional methods for sweetening natural gas (NG) is to remove hydrogen sulfide (H2S) and significantly lower carbon dioxide (CO2). However, when NG processes are integrated into the carbon capture and storage (CCS) framework, there is potential for synergy between these two technologies. A steady-state model utilizing a hybrid solvent consisting of N-methyl-2-pyrrolidone (NMP) and monoethanolamine (MEA) has been developed to successfully anticipate the CO2 and H2S capture process from NG. The model was tested against important variables affecting process performance. This article specifically explores the impact of operational parameters such as lean amine temperature, absorber pressure, and amine flow rate on the concentrations of CO2 and H2S in the sweet gas and reboiler duty. The result shows that hybrid solvents (MEA + NMP) perform better in removing acid gases and reducing reboiler duty than conventional chemical solvent MEA. The primary purpose is to meet product requirements while consuming the least energy possible, which is in line with any process plant's efficiency goals.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Gao W.; Liang S.; Wang R.; Jiang Q.; Zhang Y.; Zheng Q.; Xie B.; Toe C. Y.; Zhu X.; Wang J.; Huang L.; Gao Y.; Wang Z.; Jo C.; Wang Q.; Wang L.; Liu Y.; Louis B.; Scott J.; Roger A. C.; Amal R.; He H.; Park S. E. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2020, 49 (23), 8584–8686. 10.1039/D0CS00025F. - DOI - PubMed
-
- Qiao W.; Lu H.; Zhou G.; Azimi M.; Yang Q.; Tian W. A Hybrid Algorithm for Carbon Dioxide Emissions Forecasting Based on Improved Lion Swarm Optimizer. J. Cleaner Prod. 2020, 244, 11861210.1016/j.jclepro.2019.118612. - DOI
-
- Zahid U.; Al Rowaili F. N.; Ayodeji M. K.; Ahmed U. Simulation and Parametric Analysis of CO2 Capture from Natural Gas Using Diglycolamine. Int. J. Greenhouse Gas Control 2017, 57, 42–51. 10.1016/j.ijggc.2016.12.016. - DOI
-
- Tan L. S.; Lau K. K.; Bustam M. A.; Shariff A. M. Removal of High Concentration CO2 from Natural Gas at Elevated Pressure via Absorption Process in Packed Column. J. Nat. Gas Chem. 2012, 21 (1), 7–10. 10.1016/S1003-9953(11)60325-3. - DOI
-
- Farooqi A. S.; Yusuf M.; Zabidi N. A. M.; Sanaullah K.; Abdullah B.. CO2 Conversion Technologies for Clean Fuels Production. In Carbon Dioxide Capture Convers; Elsevier, 2022; pp 37–6310.1016/B978-0-323-85585-3.00006-7. - DOI
LinkOut - more resources
Full Text Sources
