Consensus Guidance for Monitoring Individuals With Islet Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes
- PMID: 38912694
- PMCID: PMC11381572
- DOI: 10.2337/dci24-0042
Consensus Guidance for Monitoring Individuals With Islet Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes
Erratum in
-
Erratum. Consensus Guidance for Monitoring Individuals With Islet Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes. Diabetes Care 2024;47:1276-1298.Diabetes Care. 2024 Nov 1;47(11):2033. doi: 10.2337/dc24-er11a. Diabetes Care. 2024. PMID: 39240700 Free PMC article. No abstract available.
Abstract
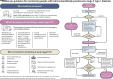
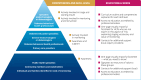
Given the proven benefits of screening to reduce diabetic ketoacidosis (DKA) likelihood at the time of stage 3 type 1 diabetes diagnosis, and emerging availability of therapy to delay disease progression, type 1 diabetes screening programs are being increasingly emphasized. Once broadly implemented, screening initiatives will identify significant numbers of islet autoantibody-positive (IAb+) children and adults who are at risk for (confirmed single IAb+) or living with (multiple IAb+) early-stage (stage 1 and stage 2) type 1 diabetes. These individuals will need monitoring for disease progression; much of this care will happen in nonspecialized settings. To inform this monitoring, JDRF, in conjunction with international experts and societies, developed consensus guidance. Broad advice from this guidance includes the following: 1) partnerships should be fostered between endocrinologists and primary care providers to care for people who are IAb+; 2) when people who are IAb+ are initially identified, there is a need for confirmation using a second sample; 3) single IAb+ individuals are at lower risk of progression than multiple IAb+ individuals; 4) individuals with early-stage type 1 diabetes should have periodic medical monitoring, including regular assessments of glucose levels, regular education about symptoms of diabetes and DKA, and psychosocial support; 5) interested people with stage 2 type 1 diabetes should be offered trial participation or approved therapies; and 6) all health professionals involved in monitoring and care of individuals with type 1 diabetes have a responsibility to provide education. The guidance also emphasizes significant unmet needs for further research on early-stage type 1 diabetes to increase the rigor of future recommendations and inform clinical care.
© 2024 by the American Diabetes Association and the European Association for the Study of Diabetes.
Conflict of interest statement
Figures

References
-
- Gorsuch AN, Spencer KM, Lister J, et al. Evidence for a long prediabetic period in type I (insulin-dependent) diabetes mellitus. Lancet 1981;2:1363–1365 - PubMed
-
- Riley WJ, Atkinson MA, Schatz DA, Maclaren NK. Comparison of islet autoantibodies in ‘pre-diabetes’ and recommendations for screening. J Autoimmun 1990;3 Suppl 1:47–51 - PubMed
-
- Hirsch JS. FDA approves teplizumab: a milestone in type 1 diabetes. Lancet Diabetes Endocrinol 2023;11:18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

