Neutrophil Heterogeneity Is Modified during Acute Lung Inflammation in Apoa1-/- Mice
- PMID: 38912868
- PMCID: PMC11300144
- DOI: 10.4049/jimmunol.2300459
Neutrophil Heterogeneity Is Modified during Acute Lung Inflammation in Apoa1-/- Mice
Abstract
Neutrophils play important roles in inflammatory airway diseases. In this study, we assessed whether apolipoprotein A-I modifies neutrophil heterogeneity as part of the mechanism by which it attenuates acute airway inflammation. Neutrophilic airway inflammation was induced by daily intranasal administration of LPS plus house dust mite (LPS+HDM) to Apoa1-/- and Apoa1+/+ mice for 3 d. Single-cell RNA sequencing was performed on cells recovered from bronchoalveolar lavage fluid on day 4. Unsupervised profiling identified 10 clusters of neutrophils in bronchoalveolar lavage fluid from Apoa1-/- and Apoa1+/+ mice. LPS+HDM-challenged Apoa1-/- mice had an increased proportion of the Neu4 neutrophil cluster that expressed S100a8, S100a9, and Mmp8 and had high maturation, aggregation, and TLR4 binding scores. There was also an increase in the Neu6 cluster of immature neutrophils, whereas neutrophil clusters expressing IFN-stimulated genes were decreased. An unsupervised trajectory analysis showed that Neu4 represented a distinct lineage in Apoa1-/- mice. LPS+HDM-challenged Apoa1-/- mice also had an increased proportion of recruited airspace macrophages, which was associated with a reciprocal reduction in resident airspace macrophages. Increased expression of a common set of proinflammatory genes, S100a8, S100a9, and Lcn2, was present in all neutrophils and airspace macrophages from LPS+HDM-challenged Apoa1-/- mice. These findings show that Apoa1-/- mice have increases in specific neutrophil and macrophage clusters in the lung during acute inflammation mediated by LPS+HDM, as well as enhanced expression of a common set of proinflammatory genes. This suggests that modifications in neutrophil and macrophage heterogeneity contribute to the mechanism by which apolipoprotein A-I attenuates acute airway inflammation.
Figures

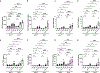
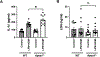





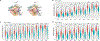

References
-
- Gernez Y, Tirouvanziam R, and Chanez P. 2010. Neutrophils in chronic inflammatory airway diseases: can we target them and how? Eur Respir J 35: 467–469. - PubMed
-
- Ng LG, Ostuni R, and Hidalgo A. 2019. Heterogeneity of neutrophils. Nat Rev Immunol 19: 255–265. - PubMed
-
- Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernandez-Calvo G, Khoyratty TE, van Grinsven E, Gonzalez-Hernandez S, Nicolas-Avila JA, Vicanolo T, Maccataio A, Benguria A, Li JL, Adrover JM, Aroca-Crevillen A, Quintana JA, Martin-Salamanca S, Mayo F, Ascher S, Barbiera G, Soehnlein O, Gunzer M, Ginhoux F, Sanchez-Cabo F, Nistal-Villan E, Schulz C, Dopazo A, Reinhardt C, Udalova IA, Ng LG, Ostuni R, and Hidalgo A. 2020. Co-option of Neutrophil Fates by Tissue Environments. Cell 183: 1282–1297 e1218. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

