The αC-β4 loop controls the allosteric cooperativity between nucleotide and substrate in the catalytic subunit of protein kinase A
- PMID: 38913408
- PMCID: PMC11196109
- DOI: 10.7554/eLife.91506
The αC-β4 loop controls the allosteric cooperativity between nucleotide and substrate in the catalytic subunit of protein kinase A
Abstract
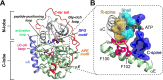
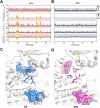
Allosteric cooperativity between ATP and substrates is a prominent characteristic of the cAMP-dependent catalytic subunit of protein kinase A (PKA-C). This long-range synergistic action is involved in substrate recognition and fidelity, and it may also regulate PKA's association with regulatory subunits and other binding partners. To date, a complete understanding of this intramolecular mechanism is still lacking. Here, we integrated NMR(Nuclear Magnetic Resonance)-restrained molecular dynamics simulations and a Markov State Model to characterize the free energy landscape and conformational transitions of PKA-C. We found that the apoenzyme populates a broad free energy basin featuring a conformational ensemble of the active state of PKA-C (ground state) and other basins with lower populations (excited states). The first excited state corresponds to a previously characterized inactive state of PKA-C with the αC helix swinging outward. The second excited state displays a disrupted hydrophobic packing around the regulatory (R) spine, with a flipped configuration of the F100 and F102 residues at the αC-β4 loop. We validated the second excited state by analyzing the F100A mutant of PKA-C, assessing its structural response to ATP and substrate binding. While PKA-CF100A preserves its catalytic efficiency with Kemptide, this mutation rearranges the αC-β4 loop conformation, interrupting the coupling of the two lobes and abolishing the allosteric binding cooperativity. The highly conserved αC-β4 loop emerges as a pivotal element to control the synergistic binding of nucleotide and substrate, explaining how mutations or insertions near or within this motif affect the function and drug sensitivity in homologous kinases.
Keywords: E. coli; allosteric mutations; binding cooperativity; cAMP-dependent kinase A; molecular biophysics; protein kinases; structural biology.
© 2023, Olivieri, Wang et al.
Conflict of interest statement
CO, YW, CW, MS, KH, DB, JG, CC, MV, ST, GV No competing interests declared
Figures




















Update of
-
The αC-β4 loop controls the allosteric cooperativity between nucleotide and substrate in the catalytic subunit of protein kinase A.bioRxiv [Preprint]. 2023 Sep 15:2023.09.12.557419. doi: 10.1101/2023.09.12.557419. bioRxiv. 2023. Update in: Elife. 2024 Jun 24;12:RP91506. doi: 10.7554/eLife.91506. PMID: 37745542 Free PMC article. Updated. Preprint.
References
-
- Best RB, Zhu X, Shim J, Lopes PEM, Mittal J, Feig M, Mackerell AD., Jr Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. Journal of Chemical Theory and Computation. 2012;8:3257–3273. doi: 10.1021/ct300400x. - DOI - PMC - PubMed
-
- Beuschlein F, Fassnacht M, Assié G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL, Wieland T, Sbiera S, Faucz FR, Schaak K, Schmittfull A, Schwarzmayr T, Barreau O, Vezzosi D, Rizk-Rabin M, Zabel U, Szarek E, Salpea P, Forlino A, Vetro A, Zuffardi O, Kisker C, Diener S, Meitinger T, Lohse MJ, Reincke M, Bertherat J, Strom TM, Allolio B. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. The New England Journal of Medicine. 2014;370:1019–1028. doi: 10.1056/NEJMoa1310359. - DOI - PMC - PubMed
-
- Biarnés X, Pietrucci F, Marinelli F, Laio A. METAGUI. A VMD interface for analyzing metadynamics and molecular dynamics simulations. Computer Physics Communications. 2012;183:203–211. doi: 10.1016/j.cpc.2011.08.020. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

