Treatment of advanced BP-NETS with lanreotide autogel/depot vs placebo: the phase III SPINET study
- PMID: 38913539
- PMCID: PMC11301421
- DOI: 10.1530/ERC-23-0337
Treatment of advanced BP-NETS with lanreotide autogel/depot vs placebo: the phase III SPINET study
Abstract
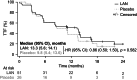
Prospective data are lacking on early somatostatin analog (SSA) therapy in bronchopulmonary neuroendocrine tumors (BP-NETs; typical carcinoids and atypical carcinoids (TCs and ACs)). SPINET (EudraCT: 2015-004992-62; NCT02683941) was a phase III, double-blind study of lanreotide autogel/depot (LAN; 120 mg every 28 days) plus best supportive care (BSC) vs placebo plus BSC, with an optional open-label treatment phase (LAN plus BSC). Patients had metastatic/unresectable, somatostatin receptor (SSTR)-positive TCs or ACs. Recruitment was stopped early owing to slow accrual; eligible patients from the double-blind phase transitioned to open-label LAN. The adapted primary endpoint was progression-free survival (PFS) during either phase for patients receiving LAN. Seventy-seven patients were randomized (LAN, n = 51 (TCs, n = 29; ACs, n = 22); placebo, n = 26 (TCs, n = 16; ACs, n = 10)). Median (95% CI) PFS during double-blind and open-label phases in patients receiving LAN was 16.6 (11.3; 21.9) months overall (primary endpoint), 21.9 (12.8, not calculable (NC)) months in TCs, and 13.8 (5.4; 16.6) months in ACs. During double-blind treatment, median (95% CI) PFS was 16.6 (11.3; 21.9) months for LAN vs 13.6 (8.3; NC) months for placebo (not significant); corresponding values were 21.9 (13.8; NC) and 13.9 (13.4; NC) months, respectively, in TCs and 13.8 (5.4; 16.6) and 11.0 (2.8; 16.9) months, respectively, in ACs. Patients' quality of life did not deteriorate and LAN was well tolerated. Although recruitment stopped early and the predefined sample size was not met, SPINET is the largest prospective study to date of SSA therapy in SSTR-positive TCs and ACs and suggests clinical benefit in TCs.
Keywords: atypical carcinoid; bronchopulmonary neuroendocrine tumors; somatostatin analog; typical carcinoid.
Conflict of interest statement
SS: honoraria – Advanced Accelerator Applications-Novartis, Ipsen, institutional grant funding – Advanced Accelerator Applications-Novartis. DF: research grant – Advanced Accelerator Applications-Novartis, Camurus, Ipsen, and Pfizer; advisory board – Advanced Accelerator Applications-Novartis, Bristol Myers Squibb, Camurus, Ipsen, Pfizer, and Sandoz; invited speaker – Advanced Accelerator Applications-Novartis, Ipsen, and Pfizer. EW: nothing to disclose. JC: scientific consultancy role (speaker and advisory roles) – Advanz, Amgen, Bayer, Esteve, Hutchinson Pharma, Ipsen, ITM, Eisai, Exelixis, Lilly, Merck Serono, Novartis, Pfizer, Roche, Sanofi; research support – AstraZeneca, Advanced Accelerator Applications – Novartis, Amgen, Bayer, Eisai, and Pfizer. WB: nothing to disclose. DR: advisory board – Advanced Accelerator Applications-Novartis; research funding – Ipsen, Merck, Novartis. MC: honoraria for speaker bureau and advisory boards – Advanced Accelerator Applications, Chiasma, Ipsen, Novartis, and Pfizer. ED: adviser for Ipsen, Novartis, and Roche. MR: honoraria – Eisai, Eli Lilly, Gilead, Ipsen, Novartis, and Roche. DH: advisory board – Advanz Pharma, Ipsen, and Novartis; invited speaker – Ipsen. EB: research grant – Advanced Accelerator Applications-Novartis, HRA, Pfizer, and Novartis; advisory board – Advanced Accelerator Applications-Novartis, HRA, Hutchison Pharma, and Novartis; principal investigator – Ipsen and Novartis; project lead – Ipsen. AH: employee and shareholder – Ipsen. XMTT: employee – Ipsen.
Figures

References
-
- Baudin E, Caplin M, Garcia-Carbonero R, Fazio N, Ferolla P, Filosso PL, Frilling A, De Herder WW, Hörsch D, Knigge U, et al.2021Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 32439–451. (10.1016/j.annonc.2021.01.003) - DOI - PubMed
-
- Bianchi A, De Marinis L, Fusco A, Lugli F, Tartaglione L, Milardi D, Mormando M, Lassandro AP, Paragliola R, Rota CA, et al.2011The treatment of neuroendocrine tumors with long-acting somatostatin analogs: a single center experience with lanreotide autogel. Journal of Endocrinological Investigation 34692–697. (10.3275/8058) - DOI - PubMed
-
- Bongiovanni A Recine F Riva N Foca F Liverani C Mercatali L Nicolini S Pieri F Amadori D & Ibrahim T. 2017Outcome analysis of first-line somatostatin analog treatment in metastatic pulmonary neuroendocrine tumors and prognostic significance of (18)FDG-PET/CT. Clinical Lung Cancer 18415–420. (10.1016/j.cllc.2016.11.004) - DOI - PubMed
-
- Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, Oberg K, Pelosi G, Perren A, Rossi RE, et al.2015Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Annals of Oncology 261604–1620. (10.1093/annonc/mdv041) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

