Accuracy of Glucagon Testing Across Transition in Young Adults With Childhood-Onset GH Deficiency
- PMID: 38913686
- PMCID: PMC11651695
- DOI: 10.1210/clinem/dgae408
Accuracy of Glucagon Testing Across Transition in Young Adults With Childhood-Onset GH Deficiency
Abstract
Context: The 2019 American Association of Clinical Endocrinologists guidelines suggested peak GH-cutoffs to glucagon test (GST) of ≤3 and ≤1 µg/L in the diagnosis of permanent GH deficiency (GHD) during the transition phase.
Objective: The aim of the study was to evaluate the accuracy of GST compared to insulin tolerance test (ITT) in the definition of GHD at adult height achievement.
Patients and methods: Ninety-seven subjects with childhood-onset GHD (median age, 17.39 years) underwent ITT, GST, and IGF-1 testing; 44 subjects were idiopathic (isolated GHD), 35 moderate organic GHD (0-2 hormone deficiencies) and 18 severe organic GHD (≥3 hormone deficiencies).
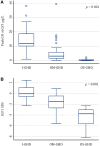
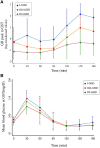
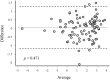
Results: Bland and Altman analysis showed a high consistency of GH peak measures after ITT and GST. Receiver operating characteristic analysis identified 7.3 μg/L as the optimal GH peak cutoff to GST [95% confidence interval (CI) 4.15-8.91; sensitivity 95.7%, specificity 88.2%, positive predictive value (PPV) 88.0%, negative predictive value (NPV) 95.7%] able to correctly classify 91.8% of the entire cohort while 5.8 μg/L was the best GH peak cutoff able to correctly classify 91.4% of moderate organic GHD patients (95% CI 3.16-7.39; sensitivity 96.0%, specificity 80.0%, PPV 92.3%, NPV 88.9%). Patients with ≥3 hormone deficiencies showed a GH peak <5 μg/L at ITT and <5.8 μg/L at GST but 1. The optimal cutoff for IGF-1 was -1.4 SD score (95% CI -1.94 to 0.77; sensitivity 75%, specificity 94%, PPV 91.7%, NPV 81.0%) that correctly classified 85.1% of the study population.
Conclusion: A GH peak to GST <5.8 μg/L represents an accurate diagnostic cutoff for young adults with childhood-onset GHD and high pretest probability of permanent GHD.
Keywords: GH deficiency; ITT; brain tumors; congenital hypopituitarism; glucagon; pituitary stalk interruption syndrome; posterior pituitary ectopia; transition; young adults.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Tauber M, Moulin P, Pienkowski C, Jouret B, Rochiccioli P. Growth hormone (GH) retesting and auxological data in 131 GH-deficient patients after completion of treatment. J Clin Endocrinol Metab. 1997;82(2):352‐356. - PubMed
-
- Maghnie M, Strigazzi C, Tinelli C, et al. Growth hormone (GH) deficiency (GHD) of childhood onset: reassessment of GH status and evaluation of the predictive criteria for permanent GHD in young adults. J Clin Endocrinol Metab. 1999;84(4):1324‐1328. - PubMed
-
- Loche S, Bizzarri C, Maghnie M, et al. Results of early reevaluation of growth hormone secretion in short children with apparent growth hormone deficiency. J Pediatr. 2002;140(4):445‐449. - PubMed
-
- Thomas M, Massa G, Maes M, et al. Growth hormone (GH) secretion in patients with childhood-onset GH deficiency: retesting after one year of therapy and at final height. Horm Res. 2003;59(1):7‐15. - PubMed
-
- Hilczer M, Smyczynska J, Stawerska R, Lewinski A. Final height and growth hormone secretion after completion of growth hormone therapy in patients with idiopathic growth hormone deficiency and with abnormalities of the hypothalamic-pituitary region. Neuro Endocrinol Lett. 2005;26(1):19‐24. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

