Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial
- PMID: 38914124
- PMCID: PMC11485243
- DOI: 10.1038/s41591-024-03133-0
Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial
Abstract
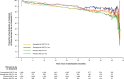
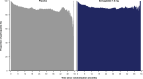
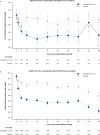
People with type 2 diabetes and chronic kidney disease have a high risk for kidney failure and cardiovascular (CV) complications. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors (SGLT2i) independently reduce CV and kidney events. The effect of combining both is unclear. FLOW trial participants with type 2 diabetes and chronic kidney disease were stratified by baseline SGLT2i use (N = 550) or no use (N = 2,983) and randomized to semaglutide/placebo. The primary outcome was a composite of kidney failure, ≥50% estimated glomerular filtration rate reduction, kidney death or CV death. The risk of the primary outcome was 24% lower in all participants treated with semaglutide versus placebo (95% confidence interval: 34%, 12%). The primary outcome occurred in 41/277 (semaglutide) versus 38/273 (placebo) participants on SGLT2i at baseline (hazard ratio 1.07; 95% confidence interval: 0.69, 1.67; P = 0.755) and in 290/1,490 versus 372/1,493 participants not taking SGLT2i at baseline (hazard ratio 0.73; 0.63, 0.85; P < 0.001; P interaction 0.109). Three confirmatory secondary outcomes were predefined. Treatment differences favoring semaglutide for total estimated glomerular filtration rate slope (ml min-1/1.73 m2/year) were 0.75 (-0.01, 1.5) in the SGLT2i subgroup and 1.25 (0.91, 1.58) in the non-SGLT2i subgroup, P interaction 0.237. Semaglutide benefits on major CV events and all-cause death were similar regardless of SGLT2i use (P interaction 0.741 and 0.901, respectively). The benefits of semaglutide in reducing kidney outcomes were consistent in participants with/without baseline SGLT2i use; power was limited to detect smaller but clinically relevant effects. ClinicalTrials.gov identifier: NCT03819153 .
© 2024. The Author(s).
Conflict of interest statement
J.F.E.M. reports grants from Novo Nordisk, the European Union and McMaster University Hamilton, Canada; consulting fees from Novo Nordisk, AstraZeneca, Bayer and Boehringer Ingelheim; honoraria from Novo Nordisk, AstraZeneca, Bayer and Novartis; and has participated on a data safety monitoring board or advisory board for AstraZeneca, Bayer, Sanofi and Boehringer Ingelheim, as well as a leadership role in the KDIGO group. P.R. has received consultancy and/or speaking fees (institutional) from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Novo Nordisk and Sanofi; and research grants from AstraZeneca, Bayer and Novo Nordisk. G.B. has been a consultant to or an executive committee member for a clinical trial: Bayer, KBP Biosciences, Ionis, Alnylam, AstraZeneca, Novo Nordisk and InREGEN; and is editor for the American Journal of Nephrology. N.B., H.B.-T., T.I. and S.R. are employees and shareholders of Novo Nordisk. R.B. has served on the speaker bureau for Novo Nordisk; and is a clinical investigator in several Novo Nordisk trials (for example, SUSTAIN6, SUSTAIN9, FLOW, SOUL and CagriSema). D.M.C. declares being a consultant for Allena Pharmaceuticals (DSMB), AstraZeneca, CSL Behring, Eli Lilly/Boehringer Ingelheim, Gilead, GSK, LG Chemical, Medtronic, Merck, Novo Nordisk, Renalytix and Zogenix; has received research funding from Amgen and Medtronic, and clinical trial support from Eli Lilly/Boehringer Ingelheim, Gilead and Novo Nordisk; has patents or royalties from UpToDate.com for authorship/editorials on reviews; has provided advisory or leadership roles for CJASN; and has received expert witness fees related to proton pump inhibitors. S.H. has received grants from Asdia, AstraZeneca, LVL, Nestle Homeperf, ISIS Diabète, Pierre Fabre and VitalAire; consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi, Servier and Valbiotis; payment or honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Bayer, Dino Santé, Eli Lilly, Novartis, Novo Nordisk, Pierre Fabre, Sanofi, Servier and Valbiotis; and support to attend meetings from AstraZeneca, Abbott, Dino Santé, Eli Lilly and Novo Nordisk. P.G. serves or has served on advisory panels for Abbott, Bayer, Boehringer Ingelheim, Janssen Pharmaceuticals, Medtronic, Novo Nordisk, Roche and Sanofi, with financial compensation for these activities received by KU Leuven; serves or has served on the speakers bureau for Abbott, Bayer, Boehringer Ingelheim, Dexcom, Insulet, Merck Sharp & Dohme, Medtronic, Novo Nordisk, Roche and VitalAire, with financial compensation for these activities received by KU Leuven; and KU Leuven has received nonfinancial support for P.G. for travel from A. Menarini Diagnostics, Medtronic, Novo Nordisk, Sanofi and Roche. J.L.G. declares funding to conduct clinical trials from AstraZeneca, Bayer, Boehringer Ingelheim and Novo Nordisk (all to the INCLIVA Research Institute); consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim and Novo Nordisk; and payment of honoraria for lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Menarini and Novo Nordisk. L.J. has received consulting/lecture fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck, MSD, Novo Nordisk and Sanofi; and payment of honoraria for lectures from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck, MSD, Novo Nordisk and Sanofi; and support to attend meetings from Bayer, Eli Lilly, Merck, MSD and Novo Nordisk. K.W.M. has received grants from AHA, Apple, Bayer, California Institute for Regenerative Medicine, CSL Behring, Eidos, Ferring, Gilead, Google (Verify), Idorsia, Johnson & Johnson, Luitpold, Novartis, PAC-12, Precordior and Sanifit; and consulting fees from Applied Therapeutics, Bayer, BMS, BridgeBio, CSL Behring, Elsevier, Fosun Pharma, Human, Johnson & Johnson, Moderna, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, and Theravance; and has equity in Human, Medeloop, Precordior, and Regencor. V.P. has received honoraria for steering committee, data monitoring committee or advisory board roles or for scientific presentations from AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, GlaxoSmithKline, Janssen, Novo Nordisk, Novartis, Otsuka, Travere, Tricida and UptoDate; and is a Board Director for George Clinical, St. Vincents Health Australia, and several independent medical research institutes. R.E.S. has received grants to the Institution from Amgen, Ablative Solution, Bayer, IPPmed, Medtronic, Novo Nordisk and Recor; and honoraria as a speaker and advisor from Ablative Solutions, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Medtronic, Merck, Novo Nordisk, Novartis, Recor, Servier and TAD. R.E.P. declares speaker fees from Lilly and Novo Nordisk; consulting fees from Bayer AG, Bayer HealthCare Pharmaceuticals, Endogenex, Gasherbrum Bio, Genprex, Getz Pharma, Intas Pharmaceuticals, Lilly, Novo Nordisk, Pfizer and Sun Pharmaceutical Industries; and research grants from Biomea Fusion, Carmot Therapeutics, Dompe, Endogenex, Fractyl, Lilly, Novo Nordisk and Sanofi. K.R.T. has received grants from Bayer and Travere Therapeutics (institutional); consulting fees and honoraria for lectures, presentations, speakers bureaus and educational events from Bayer, Boehringer Ingelheim, Eli Lilly and Company and Novo Nordisk; provides unpaid participation as the Data Safety Monitoring Board Chair for the George Clinical Institute, and NIDDK/NIH; and is also the Diabetic Kidney Disease Collaborative Chair for the American Society of Nephrology.
Figures








References
-
- Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol.9, 653–662 (2021). - PubMed
-
- Nuffield Department of Population Health Renal Studies Group. SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet400, 1788–1801 (2022). - PMC - PubMed
-
- Perkovic, V. et al. Effect of semaglutide on kidney, cardiovascular, and mortality outcomes in people with type 2 diabetes and chronic kidney disease. N. Engl. J. Med.10.1056/NEJMoa2403347 (2024).
-
- Kristensen, S. L. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol.7, 776–785 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

