Sustainable synthesis of magnetic petroleum coke/nonanyl chitosan composite for efficient removal of o-nitrophenol
- PMID: 38914588
- PMCID: PMC11196280
- DOI: 10.1038/s41598-024-64117-1
Sustainable synthesis of magnetic petroleum coke/nonanyl chitosan composite for efficient removal of o-nitrophenol
Abstract
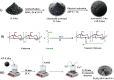
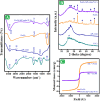
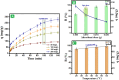
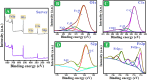
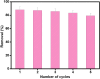
Worldwide industrialization has grown at a rapid pace, contaminating water resources, particularly with phenolic pollutants that pose a risk to aquatic systems and human health. The goal of this study is to create an inexpensive magnetic composite that can effectively remove nitrophenol (o-NP) using adsorptive means. In this instance, a nonanyl chitosan (N-Cs) derivative was synthesized and then combined with activated petroleum coke (AP-coke) and magnetic Fe3O4 to boost its adsorbability towards o-NP and to facilitate its separation. Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffractometer (XRD), Vibrating sample magnetometer (VSM), X-ray photoelectron spectroscopy (XPS), and zeta potential were employed to characterize the magnetic composite. The experimental results indicated that the Fe3O4/AP-coke/N-Cs composite possesses a greater affinity toward o-NP with a maximal efficiency reached 88% compared to 22.8, 31.2, and 45.8% for Fe3O4, AP-coke and N-Cs, respectively. The equilibrium adsorption data coincided with the Langmuir, Freundlich, and Temkin isotherm models, with a maximum adsorption capacity of 291.55 mg/g at pH 6, whereas the pseudo second order kinetic model offered the best fit to the experimental data. Besides, the developed adsorbent preserved satisfactory adsorption characteristics after reuse for five successive cycles. The proposed adsorption mechanism involves the H-bonding, π-π interaction, hydrophobic interactions and electron donor-acceptor interactions. These findings hypothesize that the constructed magnetic composite could efficiently remove nitrophenols from polluted water with high performance and ease-separation.
Keywords: Adsorption; Kinetics; Nitrophenol; Nonanyl chitosan; Petroleum coke.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ho S. Low-cost adsorbents for the removal of phenol/phenolics, pesticides, and dyes from wastewater systems: A review. Water. 2022;14:3203. doi: 10.3390/w14203203. - DOI
-
- Mukherjee S, Kumar S, Misra AK, Fan M. Removal of phenols from water environment by activated carbon, bagasse ash and wood charcoal. Chem. Eng. J. 2007;129:133–142. doi: 10.1016/j.cej.2006.10.030. - DOI
-
- Rahman EA, Hassan S, El-Subruiti G, Kamel A, Diab H. Removal of Uranium-238 ions from contaminated ground water containing NORM by adsorption on fly ash carbon: Equilibrium, kinetic and thermodynamic studies. Arab J. Nucl. Phys. Mater. Sci. Appl. 2021;54:92–103.
-
- Anku WW, Mamo MA, Govender PP. Phenolic compounds in water: sources, reactivity, toxicity and treatment methods. Phenolic Compd. Nat. Source Import. Appl. 2017 doi: 10.5772/66927. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

