Early reappearance of intraclonal proliferative subpopulations in ibrutinib-resistant chronic lymphocytic leukemia
- PMID: 38914716
- PMCID: PMC11286529
- DOI: 10.1038/s41375-024-02301-y
Early reappearance of intraclonal proliferative subpopulations in ibrutinib-resistant chronic lymphocytic leukemia
Abstract
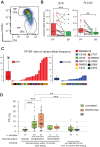
The Bruton's tyrosine kinase (BTK) inhibitor ibrutinib represents an effective strategy for treatment of chronic lymphocytic leukemia (CLL), nevertheless about 30% of patients eventually undergo disease progression. Here we investigated by flow cytometry the long-term modulation of the CLL CXCR4dim/CD5bright proliferative fraction (PF), its correlation with therapeutic outcome and emergence of ibrutinib resistance. By longitudinal tracking, the PF, initially suppressed by ibrutinib, reappeared upon early disease progression, without association with lymphocyte count or serum beta-2-microglobulin. Somatic mutations of BTK/PLCG2, detected in 57% of progressing cases, were significantly enriched in PF with a 3-fold greater allele frequency than the non-PF fraction, suggesting a BTK/PLCG2-mutated reservoir resident within the proliferative compartments. PF increase was also present in BTK/PLCG2-unmutated cases at progression, indicating that PF evaluation could represent a marker of CLL progression under ibrutinib. Furthermore, we evidence different transcriptomic profiles of PF at progression in cases with or without BTK/PLCG2 mutations, suggestive of a reactivation of B-cell receptor signaling or the emergence of bypass signaling through MYC and/or Toll-Like-Receptor-9. Clinically, longitudinal monitoring of the CXCR4dim/CD5bright PF by flow cytometry may provide a simple tool helping to intercept CLL progression under ibrutinib therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- IG-21687/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- PNRR-MAD-2022-12375673 (Next Generation EU, M6/C2_CALL 2022)/Ministero della Salute (Ministry of Health, Italy)
- RF-2018-12365790/Ministero della Salute (Ministry of Health, Italy)
- na/Associazione Italiana Contro le Leucemie - Linfomi e Mieloma (Associazione Italiana Contro le Leucemie - Linfomi e Mieloma ONLUS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

