Social determinants of health inequalities in early phase clinical trials in Northern England
- PMID: 38914804
- PMCID: PMC11333496
- DOI: 10.1038/s41416-024-02765-w
Social determinants of health inequalities in early phase clinical trials in Northern England
Abstract
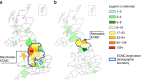
Background: Early phase clinical trials in Oncology represent a subspecialised area where UK patient selection is influenced by access to Experimental Cancer Medicine Centres (ECMCs). Equity of access with respect to social determinants of health (SDoH) were explored for two major ECMCs.
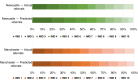
Methods: A retrospective cohort study including all referrals to Newcastle and Manchester ECMCs in 2021 was completed. Consent to screening or pre-screening was stratified against SDoH characteristics, including: Index of Multiple Deprivation (IMD) decile, ethnicity and distance to centre.
Results: 1243 patients were referred for trials. IMD quintile 1 (most deprived) patients had reduced likelihood of referral compared to expected population models (OR, 0.67; 95% CI: 0.55 to 0.80, p = <0.0001). IMD quintile 5 (least deprived) had increased likelihood of referral (OR, 1.46; 95% CI: 1.17 to 1.82, p = 0.0007). Living beyond median distance from Manchester reduced the likelihood of consenting to trials (OR, 0.72; 95% CI: 0.55 to 0.94, p = 0.015). Ethnicity data represented a White British propensity.
Conclusions: Inequalities in socioeconomic and geographic factors influence referral and enrolment to early phase clinical trials in Northern England. This has implications for equity of access and generalisability of trial results internationally and warrants further study.
© 2024. The Author(s).
Conflict of interest statement
All conflicts of interest are declared and do not directly relate to this manuscript. CO is on Pfizer, Advisory Board. MGK has received honoraria from Janssen, Roche; consulting/advisory board fees from Bayer, Ellipses Pharma, Guardant Health, Janssen, OM Pharma, Roche, Seattle Genetics; speakers fees from AstraZeneca, Janssen, Roche and research funding from Roche and Novartis for his institution. MGK has received travel, accommodation, or expenses from AstraZeneca, BerGenBio, Immutep, Janssen and Roche. FT has research funding from: GSK, Novartis. FT has personal income from advisory board/consultancy/honoraria/DSMB: BMS, GSK, T-Knife Therapeutics, Janssen, Immatics, Ixaka, Scenic Biotech, F-Star, Kite, Leucid, and Guidepoint. LC has had consultancy roles for Athenex, Bicycle Therapeutics and Boehringer Ingelheim. She has received institutional funding from Sierra Oncology, Athenex, Takeda, CellCentric, CytomX Therapeutics, Eli Lilly, Boehringer Ingelheim, Bicycle Therapeutics, Lupin Pharmaceuticals, Repare Therapeutics, ADC Therapeutics, Merck Serono and Kronos Bio. AG has received consultancy and speaker fees from: AstraZeneca, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb, Janssen/ J and J, MSD, Novartis, Pfizer, Lilly, Takeda, Guardant and Roche. AG has research funding from AstraZeneca. AG is Clinical Director for Cancer North East England Hull and Yorkshire Genomic Medicine Service. NC has research support to team from: AstraZeneca, Orion, F. Hoffmann-La Roche Ltd, Taiho, GSK, Novartis, Starpharma, Bayer, Eisai, UCB, Redx Pharmaceuticals, Stemline Therapeutics, Boehringer Ingelheim, Merck, AstraZeneca, Cancer Research UK, Orion, LOXO-Oncology. NC has received honoraria / travel support from: F. Hoffmann-La Roche Ltd. NC is on the advisory board of: F. Hoffmann-La Roche Ltd (unpaid), CUP Foundation (Jo’s Friends) (unpaid). DG has received research funding from: AstraZeneca and institutional research funding from: MSD, Codiak Biosciences, Starpharma,Faron Pharmaceuticals, Synthon, Janssen, AstraZeneca, Roche, BerGenBio, GSK, Bayer, Bicycle Therapeutics, Carrick, Taiho Pharmaceuticals, CytomX Therapeutics, RedX Pharma PLC, Eisai, Octimet, Orion Pharma, Kinex Pharmaceuticals, Boehringer Ingelheim, BMS, Turning Point Therapeutics, Immutep, Agalimmune, Kymab Ltd./Sanofi, Blueprint, Astellas, Cellcentric, UCB, Eli Lilly, Seagen, Repare Therapeutics, Timepoint Therapeutics, Astex, Stemline, Crescendo Biologics Ltd., ADC Therapeutics, Genentech, Avacta Life Sciences Ltd., Nurix Therapeutics, Chugai Pharmaceuticals, Incyte. RP has received honoria for attending advisory boards from Pierre Faber, Bayer, Novartis, BMS, Ellipses, Immunocore, Genmab, Astex Therapeutics, MSD, Nerviano, AmLo, Incyte, Cybrexa Benevolent AI and Sanofi Aventis. RP has received honoraria for working as an IDMC member for Alligator Biosciences, GSK, Onxeo, SOTIO Biotech AG, and AstraZeneca. RP has been paid for delivery of educational talks or chairing educational meetings by AstraZeneca, Novartis, Bayer, MSD and BMS. RP has received funds to support attendance at conferences from MSD and BMS.
Figures

References
-
- APPG on cancer. Cancer inequalities report - Macmillan Cancer Support. Available at: https://www.macmillan.org.uk/documents/getinvolved/campaigns/appg/britai... (Accessed: 02 September 2023).
-
- Saunders CL, Abel GA, Lyratzopoulos G. ‘Inequalities in reported cancer patient experience by socio‐demographic characteristic and cancer site: evidence from respondents to the English Cancer Patient Experience Survey’. Eur J Cancer Care. 2014;24:85–98. 10.1111/ecc.12267.10.1111/ecc.12267 - DOI - PMC - PubMed
-
- What are healthcare inequalities? NHS choices. Available at: https://www.england.nhs.uk/about/equality/equality-hub/national-healthca... (Accessed: 02 September 2023).
-
- Not ‘hard to reach’ – increasing diversity in research participation (no date) NHS choices. Available at: https://www.england.nhs.uk/blog/not-hard-to-reach-increasing-diversity-i... (Accessed: 02 September 2023).
-
- Jenkins H. Health inequalities: ‘we have a moral duty to reduce them’, Cancer Research UK - Cancer News. 2022. Available at: https://news.cancerresearchuk.org/2022/02/15/health-inequalities-we-have... (Accessed: 02 September 2023).
MeSH terms
LinkOut - more resources
Full Text Sources

