Hormone replacement therapy and cancer mortality in women with 17 site-specific cancers: a cohort study using linked medical records
- PMID: 38914805
- PMCID: PMC11333726
- DOI: 10.1038/s41416-024-02767-8
Hormone replacement therapy and cancer mortality in women with 17 site-specific cancers: a cohort study using linked medical records
Abstract
Background: There is limited evidence on the safety of Hormone Replacement Therapy (HRT) in women with cancer. Therefore, we systematically examined HRT use and cancer-specific mortality in women with 17 site-specific cancers.
Methods: Women newly diagnosed with 17 site-specific cancers from 1998 to 2019, were identified from general practitioner (GP) records, hospital diagnoses or cancer registries in Scotland, Wales and England. Breast cancer patients were excluded because HRT is contraindicated in breast cancer patients. The primary outcome was time to cancer-specific mortality. Time-dependent Cox regression models were used to calculate adjusted hazard ratios (HR) and 95% confidence intervals (95% CIs) for cancer-specific mortality by systemic HRT use.
Results: The combined cancer cohorts contained 182,589 women across 17 cancer sites. Overall 7% of patients used systemic HRT after their cancer diagnosis. There was no evidence that HRT users, compared with non-users, had higher cancer-specific mortality at any cancer site. In particular, no increase was observed in common cancers including lung (adjusted HR = 0.98 95% CI 0.90, 1.07), colorectal (adjusted HR = 0.79 95% CI 0.70, 0.90), and melanoma (adjusted HR = 0.77 95% CI 0.58, 1.02).
Conclusions: We observed no evidence of increased cancer-specific mortality in women with a range of cancers (excluding breast) receiving HRT.
© 2024. The Author(s).
Conflict of interest statement
JHC is an unpaid director of QResearch, a not-for-profit organisation which is a partnership between the University of Oxford and EMIS Health who supply the QResearch database used for this work. JHC has a 50% shareholding in ClinRisk Ltd, co-owning it with her husband, who is a director. As a shareholder and spouse of a director she has a financial and family interest in the ongoing and future success of the company. The company licences software both to the private sector and to NHS bodies or bodies that provide services to the NHS (through GP electronic health record providers, pharmacies, hospital providers and other NHS providers). This software implements algorithms (outside the scope of this research) developed from access to the QResearch database during her time at the University of Nottingham. The other authors have declared no competing interests.
Figures
References
-
- Cobin RH, Goodman NF. American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on Menopause–2017 Update. Endocr Pr. 2017;23:869–80.10.4158/EP171828.PS - DOI - PubMed
-
- Joint Formulary Committee. British National Formulary 80: September 2020-March 2021. (BMJ Group and Pharmaceutical Press: London, UK, 2020).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

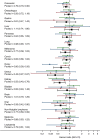
 ), Scotland (
), Scotland ( ), Wales (
), Wales ( ) and pooled (
) and pooled ( ), by site.
), by site.