Mistletoe Extract in Patients With Advanced Pancreatic Cancer: a Double-Blind, Randomized, Placebo-Controlled Tial (MISTRAL)
- PMID: 38915151
- PMCID: PMC11539882
- DOI: 10.3238/arztebl.m2024.0080
Mistletoe Extract in Patients With Advanced Pancreatic Cancer: a Double-Blind, Randomized, Placebo-Controlled Tial (MISTRAL)
Abstract
Background: Patients with advanced pancreatic cancer have limited survival and few treatment options. We studied whether mistletoe extract (ME), in addition to comprehensive oncological treatment and palliative care, prolongs overall survival (OS) and improves health-related quality of life (HRQoL).
Methods: The double-blind, placebo-controlled MISTRAL trial was conducted in Swedish oncology centers. The main inclusion criteria were advanced exocrine pancreatic cancer and Eastern Cooperative Oncology Group (ECOG) performance status 0-2. The subjects were randomly assigned to ME (n=143) or placebo (n=147) and were stratified by study site and by eligibility (yes/no) for palliative chemotherapy (June 2016-December 2021). ME or placebo was injected subcutaneously three times a week for nine months. The primary endpoint was overall survival (OS); one of the secondary endpoints was the HRQoL dimension global health/QoL (EORTC-QLQ-C30), as assessed at seven time points over nine months. Trial registration: EudraCT 2014-004552-64, NCT02948309.
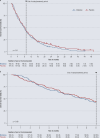
Results: No statistically significant benefit of adding ME to standard treatment was seen with respect to either OS or global health/ QoL. The adjusted hazard ratio for OS was 1.13 [0.89; 1.44], with a median survival time of 7.8 and 8.3 months for ME and placebo, respectively. The figures for the HRQoL dimension "global health/QoL" were similar in the two groups (p=0.86). The number, severity, and outcome of the reported adverse events were similar as well, except for more common local skin reactions at ME injection sites (66% vs. 1%).
Conclusion: ME is unlikely to have a clinically significant effect on OS or the HRQoL dimension global health/QoL when administered in patients with advanced pancreatic cancer in addition to comprehensive cancer care.
Figures


References
-
- Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655–663. - PubMed
-
- Rostock M. Die Misteltherapie in der Behandlung von Patienten mit einer Krebserkrankung. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2020;63:535–540. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

