This is a preprint.
Neural crest and periderm-specific requirements of Irf6 during neural tube and craniofacial development
- PMID: 38915513
- PMCID: PMC11195129
- DOI: 10.1101/2024.06.11.598425
Neural crest and periderm-specific requirements of Irf6 during neural tube and craniofacial development
Update in
-
Neural crest and periderm-specific requirements of Irf6 during neural tube and craniofacial development.Dev Biol. 2025 Jun;522:106-115. doi: 10.1016/j.ydbio.2025.03.006. Epub 2025 Mar 18. Dev Biol. 2025. PMID: 40113028 Review.
Abstract
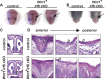
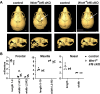
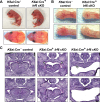
IRF6 is a key genetic determinant of syndromic and non-syndromic cleft lip and palate. The ability to interrogate post-embryonic requirements of Irf6 has been hindered, as global Irf6 ablation in the mouse causes neonatal lethality. Prior work analyzing Irf6 in mouse models defined its role in the embryonic surface epithelium and periderm where it is required to regulate cell proliferation and differentiation. Several reports have also described Irf6 gene expression in other cell types, such as muscle, and neuroectoderm. However, analysis of a functional role in non-epithelial cell lineages has been incomplete due to the severity and lethality of the Irf6 knockout model and the paucity of work with a conditional Irf6 allele. Here we describe the generation and characterization of a new Irf6 floxed mouse model and analysis of Irf6 ablation in periderm and neural crest lineages. This work found that loss of Irf6 in periderm recapitulates a mild Irf6 null phenotype, suggesting that Irf6-mediated signaling in periderm plays a crucial role in regulating embryonic development. Further, conditional ablation of Irf6 in neural crest cells resulted in an anterior neural tube defect of variable penetrance. The generation of this conditional Irf6 allele allows for new insights into craniofacial development and new exploration into the post-natal role of Irf6.
Keywords: Irf6; Van der Woude Syndrome; cleft palate; neural crest; neural tube; periderm.
Figures



Similar articles
-
Neural crest and periderm-specific requirements of Irf6 during neural tube and craniofacial development.Dev Biol. 2025 Jun;522:106-115. doi: 10.1016/j.ydbio.2025.03.006. Epub 2025 Mar 18. Dev Biol. 2025. PMID: 40113028 Review.
-
IRF6 and SPRY4 Signaling Interact in Periderm Development.J Dent Res. 2017 Oct;96(11):1306-1313. doi: 10.1177/0022034517719870. Epub 2017 Jul 21. J Dent Res. 2017. PMID: 28732181 Free PMC article.
-
IRF6 expression in basal epithelium partially rescues Irf6 knockout mice.Dev Dyn. 2017 Sep;246(9):670-681. doi: 10.1002/dvdy.24537. Epub 2017 Jul 19. Dev Dyn. 2017. PMID: 28643456 Free PMC article.
-
Altered regulation of cell migration in IRF6-mutated orofacial cleft patients-derived primary cells reveals a novel role of Rho GTPases in cleft/lip palate development.Cells Dev. 2021 Jun;166:203674. doi: 10.1016/j.cdev.2021.203674. Epub 2021 Mar 17. Cells Dev. 2021. PMID: 33994351
-
Non-random distribution of deleterious mutations in the DNA and protein-binding domains of IRF6 are associated with Van Der Woude syndrome.Mol Genet Genomic Med. 2020 Aug;8(8):e1355. doi: 10.1002/mgg3.1355. Epub 2020 Jun 17. Mol Genet Genomic Med. 2020. PMID: 32558391 Free PMC article. Review.
References
-
- Botti E., Spallone G., Moretti F., Marinari B., Pinetti V., Galanti S., De Meo P.D., De Nicola F., Ganci F., Castrignano T., et al. (2011). Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proceedings of the National Academy of Sciences of the United States of America 108, 13710–13715. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
