This is a preprint.
Endothelial cell Piezo1 promotes vascular smooth muscle cell differentiation on large arteries
- PMID: 38915529
- PMCID: PMC11195244
- DOI: 10.1101/2024.06.11.598539
Endothelial cell Piezo1 promotes vascular smooth muscle cell differentiation on large arteries
Update in
-
Endothelial cell Piezo1 promotes vascular smooth muscle cell differentiation on large arteries.Eur J Cell Biol. 2025 Mar;104(1):151473. doi: 10.1016/j.ejcb.2024.151473. Epub 2024 Dec 20. Eur J Cell Biol. 2025. PMID: 39729736
Abstract
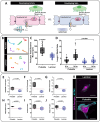
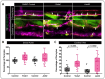
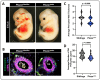
Vascular stabilization is a mechanosensitive process, in part driven by blood flow. Here, we demonstrate the involvement of the mechanosensitive ion channel, Piezo1, in promoting arterial accumulation of vascular smooth muscle cells (vSMCs) during zebrafish development. Using a series of small molecule antagonists or agonists to temporally regulate Piezo1 activity, we identified a role for the Piezo1 channel in regulating klf2a levels and altered targeting of vSMCs between arteries and veins. Increasing Piezo1 activity suppressed klf2a and increased vSMC association with the cardinal vein, while inhibition of Piezo1 activity increased klf2a levels and decreased vSMC association with arteries. We supported the small molecule data with in vivo genetic suppression of piezo1 and 2 in zebrafish, resulting in loss of transgelin+ vSMCs on the dorsal aorta. Further, endothelial cell (EC)-specific Piezo1 knockout in mice was sufficient to decrease vSMC accumulation along the descending dorsal aorta during development, thus phenocopying our zebrafish data, and supporting functional conservation of Piezo1 in mammals. To determine mechanism, we used in vitro modeling assays to demonstrate that differential sensing of pulsatile versus laminar flow forces across endothelial cells changes the expression of mural cell differentiation genes. Together, our findings suggest a crucial role for EC Piezo1 in sensing force within large arteries to mediate mural cell differentiation and stabilization of the arterial vasculature.
Keywords: Piezo1; arterial stabilization; mural cell; vascular smooth muscle; zebrafish.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



References
-
- Rahman M., and Siddik A.B. (2024). Anatomy, Arterioles. In StatPearls. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
