This is a preprint.
Neuronal identity control at the resolution of a single transcription factor isoform
- PMID: 38915533
- PMCID: PMC11195191
- DOI: 10.1101/2024.06.14.598883
Neuronal identity control at the resolution of a single transcription factor isoform
Abstract
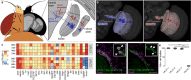
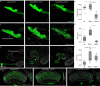
The brain exhibits remarkable neuronal diversity which is critical for its functional integrity. From the sheer number of cell types emerging from extensive transcriptional, morphological, and connectome datasets, the question arises of how the brain is capable of generating so many unique identities. 'Terminal selectors' are transcription factors hypothesized to determine the final identity characteristics in post-mitotic cells. Which transcription factors function as terminal selectors and the level of control they exert over different terminal characteristics are not well defined. Here, we establish a novel role for the transcription factor broad as a terminal selector in Drosophila melanogaster. We capitalize on existing large sequencing and connectomics datasets and employ a comprehensive characterization of terminal characteristics including Perturb-seq and whole-cell electrophysiology. We find a single isoform broad-z4 serves as the switch between the identity of two visual projection neurons LPLC1 and LPLC2. Broad-z4 is natively expressed in LPLC1, and is capable of transforming the transcriptome, morphology, and functional connectivity of LPLC2 cells into LPLC1 cells when perturbed. Our comprehensive work establishes a single isoform as the smallest unit underlying an identity switch, which may serve as a conserved strategy replicated across developmental programs.
Keywords: Cell Identity; Drosophila melanogaster; Electrophysiology; Neurobiology; Neurodevelopment; Terminal Selector; Transcription Factors; Visual Projection Neurons; Visual System; scRNA-seq.
Figures



References
-
- Ahn S., Yang H., Son S., Lee H. S., Park D., Yim H., Choi H. J., Swoboda P., & Lee J. (2022). The C. elegans regulatory factor X (RFX) DAF-19M module: A shift from general ciliogenesis to cell-specific ciliary and behavioral specialization. Cell Rep, 39(2), 110661. 10.1016/j.celrep.2022.110661 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
