This is a preprint.
Plasma proteomics in the UK Biobank reveals youthful brains and immune systems promote healthspan and longevity
- PMID: 38915561
- PMCID: PMC11195058
- DOI: 10.1101/2024.06.07.597771
Plasma proteomics in the UK Biobank reveals youthful brains and immune systems promote healthspan and longevity
Abstract
Organ-derived plasma protein signatures derived from aptamer protein arrays track organ-specific aging, disease, and mortality in humans, but the robustness and clinical utility of these models and their biological underpinnings remain unknown. Here, we estimate biological age of 11 organs from 44,526 individuals in the UK Biobank using an antibody-based proteomics platform to model disease and mortality risk. Organ age estimates are associated with future onset of heart failure (heart age HR=1.83), chronic obstructive pulmonary disease (lung age HR=1.39), type II diabetes (kidney age HR=1.58), and Alzheimer's disease (brain age HR=1.81) and sensitive to lifestyle factors such as smoking and exercise, hormone replacement therapy, or supplements. Remarkably, the accrual of aged organs progressively increases mortality risk while a youthful brain and immune system are uniquely associated with disease-free longevity. These findings support the use of plasma proteins for monitoring organ health and the efficacy of drugs targeting organ aging disease.
Conflict of interest statement
T.W-C., H.S.O. and J.R. are co-founders and scientific advisors of Teal Omics Inc. and have received equity stakes. T.W.-C. is a co-founder and scientific advisor of Alkahest Inc. and Qinotto Inc. and has received equity stakes in these companies.
Figures

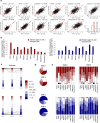
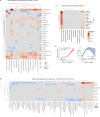







References
-
- López-Otín C., Blasco M. A., Partridge L., Serrano M. & Kroemer G. Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023). - PubMed
-
- Tian Y. E. et al. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat. Med. 29, 1221–1231 (2023). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
