This is a preprint.
A suite of enhancer AAVs and transgenic mouse lines for genetic access to cortical cell types
- PMID: 38915722
- PMCID: PMC11195086
- DOI: 10.1101/2024.06.10.597244
A suite of enhancer AAVs and transgenic mouse lines for genetic access to cortical cell types
Update in
-
A suite of enhancer AAVs and transgenic mouse lines for genetic access to cortical cell types.Cell. 2025 May 29;188(11):3045-3064.e23. doi: 10.1016/j.cell.2025.05.002. Epub 2025 May 21. Cell. 2025. PMID: 40403729
Abstract
The mammalian cortex is comprised of cells classified into types according to shared properties. Defining the contribution of each cell type to the processes guided by the cortex is essential for understanding its function in health and disease. We used transcriptomic and epigenomic cortical cell type taxonomies from mouse and human to define marker genes and putative enhancers and created a large toolkit of transgenic lines and enhancer AAVs for selective targeting of cortical cell populations. We report evaluation of fifteen new transgenic driver lines, two new reporter lines, and >800 different enhancer AAVs covering most subclasses of cortical cells. The tools reported here as well as the scaled process of tool creation and modification enable diverse experimental strategies towards understanding mammalian cortex and brain function.
Keywords: AAV; Cell type; Enhancer; Epigenetics; Human; Mouse; Neocortex; Recombinase; Transcriptomics; Transgenic.
Figures



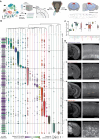
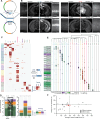
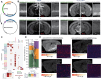


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
