CRISPR-Cas9 for selective targeting of somatic mutations in pancreatic cancers
- PMID: 38915758
- PMCID: PMC11195629
- DOI: 10.1093/narcan/zcae028
CRISPR-Cas9 for selective targeting of somatic mutations in pancreatic cancers
Abstract
Somatic mutations are desirable targets for selective elimination of cancer, yet most are found within noncoding regions. We have adapted the CRISPR-Cas9 gene editing tool as a novel, cancer-specific killing strategy by targeting the subset of somatic mutations that create protospacer adjacent motifs (PAMs), which have evolutionally allowed bacterial cells to distinguish between self and non-self DNA for Cas9-induced double strand breaks. Whole genome sequencing (WGS) of paired tumor minus normal (T-N) samples from three pancreatic cancer patients (Panc480, Panc504, and Panc1002) showed an average of 417 somatic PAMs per tumor produced from single base substitutions. Further analyses of 591 paired T-N samples from The International Cancer Genome Consortium found medians of ∼455 somatic PAMs per tumor in pancreatic, ∼2800 in lung, and ∼3200 in esophageal cancer cohorts. Finally, we demonstrated 69-99% selective cell death of three targeted pancreatic cancer cell lines using 4-9 sgRNAs designed using the somatic PAM discovery approach. We also showed no off-target activity from these tumor-specific sgRNAs in either the patient's normal cells or an irrelevant cancer using WGS. This study demonstrates the potential of CRISPR-Cas9 as a novel and selective anti-cancer strategy, and supports the genetic targeting of adult cancers.
© The Author(s) 2024. Published by Oxford University Press on behalf of NAR Cancer.
Figures
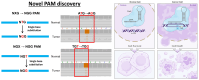

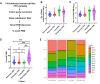
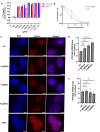


Update of
-
CRISPR-Cas9 for selective targeting of somatic mutations in pancreatic cancers.bioRxiv [Preprint]. 2023 Oct 10:2023.04.15.537042. doi: 10.1101/2023.04.15.537042. bioRxiv. 2023. Update in: NAR Cancer. 2024 Jun 19;6(2):zcae028. doi: 10.1093/narcan/zcae028. PMID: 37131822 Free PMC article. Updated. Preprint.
References
-
- Khurana E., Fu Y., Chakravarty D., Demichelis F., Rubin M.A., Gerstein M. Role of non-coding sequence variants in cancer. Nat. Rev. Genet. 2016; 17:93–108. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

