A phase 1 proof-of-concept study evaluating safety, tolerability, and biological marker responses with combination therapy of CTLA4-Ig and interleukin-2 in amyotrophic lateral sclerosis
- PMID: 38915796
- PMCID: PMC11195540
- DOI: 10.3389/fneur.2024.1415106
A phase 1 proof-of-concept study evaluating safety, tolerability, and biological marker responses with combination therapy of CTLA4-Ig and interleukin-2 in amyotrophic lateral sclerosis
Abstract
Objective: To determine whether a combination therapy with abatacept (CTLA4-Ig) and interleukin-2 (IL-2) is safe and suppresses markers of oxidative stress, inflammation, and degeneration in ALS.
Methods: In this open-label study, four participants with ALS received subcutaneous injections of low dose IL-2 (1 × 106 IU/injection/day) for 5 consecutive days every 2 weeks and one subcutaneous injection of CTLA4-Ig (125 mg/mL/injection) every 2 weeks coinciding with the first IL-2 injection of each treatment cycle. Participants received a total of 24 treatment cycles during the first 48 weeks in this 56-week study. They were closely monitored for treatment-emergent adverse events (TEAEs) and disease progression with the ALSFRS-R. Phenotypic changes within T cell populations and serum biological markers of oxidative stress [4-hydroxynonenal (4-HNE) and oxidized-LDL (ox-LDL)], inflammation (IL-18), and structural neuronal degeneration [neurofilament light chain (Nf-L)] were assessed longitudinally.
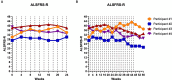
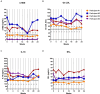
Results: CTLA4-Ig/IL-2 therapy was safe and well-tolerated in all four participants over the 56-week study. During the first 24 weeks, the average rate of change in the ALSFRS-R was +0.04 points/month. Over the 48-week treatment period, the average rate of change was -0.13 points/month with one participant improving by 0.9 points/month while the other three participants experienced an average decrease of -0.47 points/month, which is slower than the average - 1.1 points/month prior to initiation of therapy. Treg suppressive function and numbers increased during treatment. Responses in the biological markers during the first 16 weeks coincided with minimal clinical progression. Mean levels of 4-HNE decreased by 30%, ox-LDL decreased by 19%, IL-18 decreased by 23%, and Nf-L remained the same, on average, in all four participants. Oxidized-LDL levels decreased in all four participants, 4-HNE and IL-18 levels decreased in three out of four participants, and Nf-L decreased in two out of four participants.
Conclusion: The combination therapy of CTLA4-Ig and IL-2 in ALS is safe and well-tolerated with promising results of clinical efficacy and suppression of biomarkers of oxidative stress, neuroinflammation and neuronal degeneration. In this open-label study, the efficacy as measured by the ALSFRS-R and corresponding biomarkers suggests the therapeutic potential of this treatment and warrants further study in a phase 2 double-blind, placebo-controlled trial.
Clinical trial registration: ClinicalTrials.gov, NCT06307301.
Keywords: CTLA4-Ig; amyotrophic lateral scelerosis; clinical trial; interleukin-2 (IL-2); lipid perodixation; neuroinflammation; oxidative stress; phase 1.
Copyright © 2024 Thonhoff, Beers, Zhao, Faridar, Thome, Wen, Zhang, Wang and Appel.
Conflict of interest statement
The combination therapy of CTLA4-Ig and IL-2 has been licensed from the Houston Methodist Research Institute to Coya Therapeutics, Inc. JT, DB, AF, and SA are listed as inventors in a patent application. SA also serves as scientific consultant for Mitsubishi Tanabe Pharma, Eledon, and UCB Biopharma, and as Chair of the Scientific Advisory Board for Coya Therapeutics, Inc. Coya Therapeutics was not involved in the study concept or design, or acquisition, analysis, and interpretation of data, or drafting of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Thonhoff JR, Berry JD, Macklin EA, Beers DR, Mendoza PA, Zhao W, et al. . Combined regulatory T-lymphocyte and IL-2 treatment is safe, tolerable, and biologically active for 1 year in persons with amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm. (2022) 9:1–11. doi: 10.1212/NXI.0000000000200019, PMID: - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

