Diastereoselective Synthesis of the HIV Protease Inhibitor Darunavir and Related Derivatives via a Titanium Tetrachloride-Mediated Asymmetric Glycolate Aldol Addition Reaction
- PMID: 38916048
- PMCID: PMC11232028
- DOI: 10.1021/acs.joc.4c01057
Diastereoselective Synthesis of the HIV Protease Inhibitor Darunavir and Related Derivatives via a Titanium Tetrachloride-Mediated Asymmetric Glycolate Aldol Addition Reaction
Abstract
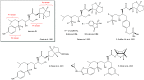
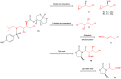
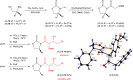
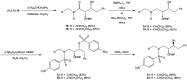
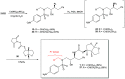
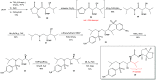
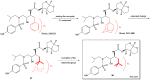
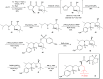
Darunavir is a potent HIV protease inhibitor that has been established as an effective tool in the fight against the progression of HIV/AIDS in the global community. The successful application of this drug has spurred the development of derivatives wherein strategic regions (e.g., P1, P1', P2, and P2') of the darunavir framework have been structurally modified. An alternate route for the synthesis of darunavir and three related P1 and P1' derivatives has been developed. This synthetic pathway involves the use of a Crimmins titanium tetrachloride-mediated oxazolidine-2-thione-guided asymmetric glycolate aldol addition reaction. The resultant aldol adduct introduces the P1 fragment of darunavir via an aldehyde. Transamidation with a selected amine (isobutylamine or 2-ethyl-1-butylamine) to cleave the auxiliary yields an amide wherein the P1' component is introduced. From this stage, the amide is reduced to the corresponding β-amino alcohol and the substrate is then bis-nosylated to introduce the requisite p-nitrobenzenesulfonamide component and activate the secondary alcohol for nucleophilic substitution. Treatment with sodium azide yielded the desired azides, and the deprotection of the p-methoxyphenoxy group is achieved with the use of ceric ammonium nitrate. Finally, hydrogenation to reduce both the aniline and azide functionalities with concurrent acylation yields darunavir and its derivatives.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- MacArthur R. D. Darunavir: promising initial results. Lancet 2007, 369, 1143–1144. 10.1016/S0140-6736(07)60499-1. - DOI - PubMed
- Ghosh A. K.; Kincaid J. F.; Choi W.; Walters D. E.; Krishnan K.; Hussain K. A.; Koo Y.; Cho H.; Rudall C.; Holland L.; et al. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bio. Med. Chem. Lett. 1998, 8, 687–690. 10.1016/S0960-894X(98)00098-5. - DOI - PubMed
-
- Koh Y.; Nakata H.; Maeda K.; Ogata H.; Bilcer G.; Devasamudram T.; Kincaid J. F.; Boross P.; Wang Y.-F.; Tie Y.; Volarath P.; Gaddis L.; Harrison R. W.; Weber I. T.; Ghosh A. K.; Mitsuya H. Novel bis -Tetrahydrofuranylurethane-Containing Nonpeptidic Protease Inhibitor (PI) UIC-94017 (TMC114) with Potent Activity against Multi-PI-Resistant Human Immunodeficiency Virus In Vitro. Antimicrob. Agents Chemother. 2003, 47, 3123–3129. 10.1128/AAC.47.10.3123-3129.2003. - DOI - PMC - PubMed
-
- Montaner J. S.; Lima V. D.; Barrios R.; Yip B.; Wood E.; Kerr T.; Shannon K.; Harrigan P. R.; Hogg R. S.; Daly P.; et al. Association of Highly Active Antiretroviral Therapy Coverage, Population Viral Load, and Yearly New HIV Diagnoses in British Columbia, Canada: A Population-Based Study. Lancet 2010, 376 (9740), 532–539. 10.1016/S0140-6736(10)60936-1. - DOI - PMC - PubMed
-
-
″Drug Approval Package: Prezista (Darumavir) NDA #021976″. U.S. Food and Drug Administration (FDA). 6 September 2006.
-
″Prezista EPAR″. European Medicines Agency (EMA). 17 September 2018.
-
″FDA Approves New HIV Treatment for Patients Who Do Not Respond to Existing Drugs″. US FDA (Press release). Archived from the original on 13 November 2016.
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

