Exploration of Immune-Modulatory Effects of Amivantamab in Combination with Pembrolizumab in Lung and Head and Neck Squamous Cell Carcinoma
- PMID: 38916448
- PMCID: PMC11253790
- DOI: 10.1158/2767-9764.CRC-24-0107
Exploration of Immune-Modulatory Effects of Amivantamab in Combination with Pembrolizumab in Lung and Head and Neck Squamous Cell Carcinoma
Abstract
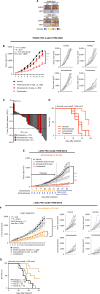
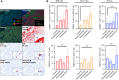
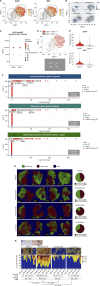
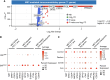
Immune checkpoint inhibitors are effective first-line therapy for solid cancers. However, low response rate and acquired resistance over time has led to the need for additional therapeutic options. Here, we evaluated synergistic antitumor efficacy of EGFR × MET targeting bispecific antibody, amivantamab with PD-L1 immunotherapy, pembrolizumab in head and neck squamous cell carcinoma (HNSCC) and lung squamous cell carcinoma tumor-bearing humanized patient-derived xenograft (PDX) models. We demonstrated that pembrolizumab or amivantamab alone was ineffective and that combination treatment induced a significant reduction of tumor growth in both models (P < 0.0001 and P < 0.01, respectively). It appeared that combination of amivantamab and pembrolizumab significantly enhanced infiltration of granzyme B-producing CD8 T cells was in the TME of HNSCC PDX (P < 0.01) and enhanced neoantigen-associated central memory CD8 T cells in circulating immune cells. Analysis of single-cell RNA transcriptomics suggested that the tumor cells dramatically upregulated EGFR and MET in response to PD-L1 immunotherapy, potentially creating a metabolic state fit for tumor persistence in the tumor microenvironment (TME) and rendered pembrolizumab ineffective. We demonstrated that EGFRHIGHMETHIGH subcluster displayed an increased expression of genes implicated in production of lactate [SLC16A3 and lactate dehydrogenase A (LDHA)] compared to the EGFRLOWMETLOW cluster. Accumulation of lactate in the TME has been associated with immunosuppression by hindering the infiltration of tumor killing CD8 T and NK cells. This study proved that amivantamab reduced glycolytic markers in the EGFRHIGHMETHIGH subcluster including SLC16A3 and LDHA and highlighted remodeling of the TME by combination treatment, providing rationale for additional therapy of amivantamab with PD-1 immunotherapy.
Significance: Amivantamab in synergy with pembrolizumab effectively eradicated EGFRHIGHMETHIGH tumor subcluster in the tumor microenvironment of head and neck squamous cell carcinoma and overcame resistance against anti-PD-1 immunotherapy.
©2024 The Authors, Published by the American Association for Cancer Research.
Conflict of interest statement
J.C. Curtin reports a patent to PCT/IB2024/052394 pending. B. Patel reports other from Johnson and Johnson outside the submitted work. B.C. Cho reports personal fees from Champions Oncology, Crown Bioscience, Imagen, PearlRiver Bio GmbH, Abion, BeiGene, Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, BMS, CJ, CureLogen, Cyrus therapeutics, Ono, Onegene Biotechnology, Yuhan, Pfizer, Eli Lilly, GI-Cell, Guardant, HK Inno-N, Imnewrun Biosciences Inc., Janssen, Takeda, MSD, Janssen, Medpacto, Blueprint medicines, RandBio, Hanmi, KANAPH Therapeutic Inc, Bridgebio therapeutics, Cyrus therapeutics, Guardant Health, Oscotec Inc, J INTS Bio, Therapex Co., Ltd, Gliead, Amgen, TheraCanVac Inc, Gencurix Inc, Bridgebio Therapeutics, KANAPH Therapeutic Inc, Cyrus Therapeutics, Interpark Bio Convergence Corp., and J INTS BIO; grants from MOGAM Institute, LG Chem, Oscotec, Interpark Bio Convergence Corp, GIInnovation, GI-Cell, Abion, AbbVie, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Champions Onoclogy, CJ bioscience, CJ Blossom Park, Cyrus, Dizal Pharma, Genexine, Janssen, Lilly, MSD, Novartis, Nuvalent, Oncternal, Ono, Regeneron, Dong-A ST, Bridgebio therapeutics, Yuhan, ImmuneOncia, Illumina, Kanaph therapeutics, Therapex, JINTSbio, Hanmi, CHA Bundang Medical Center, and Vertical Bio AG; other from DAAN Biotherapeutics, and personal fees from J INTS BIO outside the submitted work. No other disclosures were reported.
Figures


Similar articles
-
Efficacy of anti-LAG3 and anti-PD-1 combination checkpoint inhibitor therapy against head and neck squamous cell carcinoma in a genetically engineered mouse model.Oncoimmunology. 2025 Dec;14(1):2477872. doi: 10.1080/2162402X.2025.2477872. Epub 2025 Mar 17. Oncoimmunology. 2025. PMID: 40098377
-
Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck.J Immunother Cancer. 2021 May;9(5):e002088. doi: 10.1136/jitc-2020-002088. J Immunother Cancer. 2021. PMID: 33986123 Free PMC article.
-
Alum-anchored IL-12 combined with cytotoxic chemotherapy and immune checkpoint blockade enhanced antitumor immune responses in head and neck cancer models.J Immunother Cancer. 2024 Oct 23;12(10):e009712. doi: 10.1136/jitc-2024-009712. J Immunother Cancer. 2024. PMID: 39448201 Free PMC article.
-
Safety evaluation of pembrolizumab for treating recurrent head and neck squamous cell carcinoma.Expert Opin Drug Saf. 2020 Aug;19(8):927-934. doi: 10.1080/14740338.2020.1775811. Epub 2020 Jun 11. Expert Opin Drug Saf. 2020. PMID: 32458764 Review.
-
Immunotherapy in recurrent and or metastatic squamous cell carcinoma of the head and neck.Curr Opin Oncol. 2019 May;31(3):146-151. doi: 10.1097/CCO.0000000000000522. Curr Opin Oncol. 2019. PMID: 30893146 Review.
Cited by
-
Future investigative directions for novel therapeutic targets in head and neck cancer.Expert Rev Anticancer Ther. 2024 Nov;24(11):1067-1084. doi: 10.1080/14737140.2024.2417038. Epub 2024 Oct 16. Expert Rev Anticancer Ther. 2024. PMID: 39412140 Review.
-
Immune-metabolic crosstalk in HNSCC: mechanisms and therapeutic opportunities.Front Oncol. 2025 May 15;15:1553284. doi: 10.3389/fonc.2025.1553284. eCollection 2025. Front Oncol. 2025. PMID: 40444100 Free PMC article. Review.
-
Understanding and overcoming multidrug resistance in cancer.Nat Rev Clin Oncol. 2025 Jul 29. doi: 10.1038/s41571-025-01059-1. Online ahead of print. Nat Rev Clin Oncol. 2025. PMID: 40731166 Review.
References
-
- Sanmamed MF, Berraondo P, Rodriguez-Ruiz ME, Melero I. Charting roadmaps towards novel and safe synergistic immunotherapy combinations. Nat Cancer 2022;3:665–80. - PubMed
-
- Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 2017;18:e731-41. - PubMed
-
- Aguiar PN Jr, Santoro IL, Tadokoro H, de Lima Lopes G, Filardi BA, Oliveira P, et al. . The role of PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: a network meta-analysis. Immunotherapy 2016;8:479–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

