Donor types and outcomes of transplantation in myelofibrosis: a CIBMTR study
- PMID: 38916866
- PMCID: PMC11372592
- DOI: 10.1182/bloodadvances.2024013451
Donor types and outcomes of transplantation in myelofibrosis: a CIBMTR study
Abstract
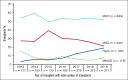
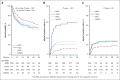
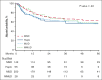
We evaluate the impact of donor types on outcomes of hematopoietic cell transplantation (HCT) in myelofibrosis, using the Center for International Blood and Marrow Transplant Research registry data for HCTs done between 2013 and 2019. In all 1597 patients, the use of haploidentical donors increased from 3% in 2013 to 19% in 2019. In study-eligible 1032 patients who received peripheral blood grafts for chronic-phase myelofibrosis, 38% of recipients of haploidentical HCT were non-White/Caucasian. Matched sibling donor (MSD)-HCTs were associated with superior overall survival (OS) in the first 3 months (haploidentical hazard ratio [HR], 5.80 [95% confidence interval (CI), 2.52-13.35]; matched unrelated (MUD) HR, 4.50 [95% CI, 2.24-9.03]; mismatched unrelated HR, 5.13 [95% CI, 1.44-18.31]; P < .001). This difference in OS aligns with lower graft failure with MSD (haploidentical HR, 6.11 [95% CI, 2.98-12.54]; matched unrelated HR, 2.33 [95% CI, 1.20-4.51]; mismatched unrelated HR, 1.82 [95% CI, 0.58-5.72]). There was no significant difference in OS among haploidentical, MUD, and mismatched unrelated donor HCTs in the first 3 months. Donor type was not associated with differences in OS beyond 3 months after HCT, relapse, disease-free survival, or OS among patients who underwent HCT within 24 months of diagnosis. Patients who experienced graft failure had more advanced disease and commonly used nonmyeloablative conditioning. Although MSD-HCTs were superior, there is no significant difference in HCT outcomes from haploidentical and MUDs. These results establish haploidentical HCT with posttransplantation cyclophosphamide as a viable option in myelofibrosis, especially for ethnic minorities underrepresented in the donor registries.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: T.J. reports receiving institutional research support from CTI BioPharma, Kartos Therapeutics, Incyte, and Bristol Myers Squibb (BMS); and advisory board participation with CareDx, BMS, Incyte, AbbVie, CTI, Kite, Cogent Biosciences, Blueprint Medicine, Telios Pharma, Protagonist Therapeutics, TScan Therapeutics, Karyopharm, and MorphoSys. R.K. reports advisory board participation for Vertex Pharmaceuticals. M.A.C. reports becoming an AstraZeneca employee while on Hospital del Mar; and advisory board or consulting fees from Novartis, Pfizer, BMS, Takeda, and Sanofi. H.E. reports advisory board for Shoreline Biosciences; and research funding from BMS. T.B. reports advisory board fees from Pfizer, Takeda, and MorphoSys. K.B. reports receiving research funding to her institution from Stemline Therapeutics. V.R.B. reports participating in safety monitoring committee for Protagonist, serving as an associate editor for the journal Current Problems in Cancer and as a contributor for BMJ Best Practice; consulting fees from Taiho, Sanofi, Imugene, Genentech, Incyte, Servier Pharmaceuticals LLC, and AbbVie; research funding (institutional) from MEI Pharma, Actinium Pharmaceutical, Sanofi US Services, AbbVie, Pfizer, Incyte, Jazz, and National Marrow Donor Program; and drug support (institutional) from Chimerix for a trial. Z.D. reports research support from Incyte, Corp, REGiMMUNE, Corp, and Taiho Oncology, Inc; and consulting fees from Sanofi, Incyte, Corp, MorphoSys AG, Inhibrx, PharmaBiome AG, and Ono Pharmaceutical. N.F. has been an advisory board member for Incyte; received speaker fees from Incyte; is on the data safety monitoring committee for Chronic Graft-versus-Host Disease Consortium; and is the medical monitor for Blood and Marrow Transplant Clinical Trial Network. A.P.G. reports advisory board/research funding from Orca Bio and Jasper Therapeutics. U.G. reports consulting and speaker bureau fees from Incyte. M.R.G. reports consulting fees from AbbVie, Amgen, Astellas, Blueprint Medicines, BMS, Cardinal Health, Sobi/CTI BioPharma, Daiichi Sankyo, Gamida Cell, Genentech, Gilead Sciences, GlaxoSmithKline (GSK)/Sierra Oncology, Incyte, Invitae, Jazz, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Servier/Agios, and Stemline Therapeutics; research support from Incyte and Janssen; and stock ownership in Medtronic. N.H. reports advisory board participation with Novartis, Gilead, Janssen, AbbVie, Pfizer, Incyte, Roche, Takeda, Jazz Pharmaceuticals, Link Pharmaceuticals, and Mallinckrodt
Figures
References
-
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. - PubMed
-
- Harrison C, Kremyanskaya M, Bose P, et al. Pelabresib (CPI-0610) as add-on to ruxolitinib in myelofibrosis: durability of response and safety beyond week 24 in the phase 2 MANIFEST Study. Blood. 2022;140(suppl 1):9659–9662.
-
- Verstovsek S, Gerds AT, Vannucchi AM, et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis (MOMENTUM): results from an international, double-blind, randomised, controlled, phase 3 study. Lancet. 2023;401(10373):269–280. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources