Ketamine vs Electroconvulsive Therapy for Treatment-Resistant Depression: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 38916891
- PMCID: PMC11200139
- DOI: 10.1001/jamanetworkopen.2024.17786
Ketamine vs Electroconvulsive Therapy for Treatment-Resistant Depression: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: The ELEKT-D: Electroconvulsive Therapy (ECT) vs Ketamine in Patients With Treatment Resistant Depression (TRD) (ELEKT-D) trial demonstrated noninferiority of intravenous ketamine vs ECT for nonpsychotic TRD. Clinical features that can guide selection of ketamine vs ECT may inform shared decision-making for patients with TRD.
Objective: To evaluate whether selected clinical features were associated with differential improvement with ketamine vs ECT.
Design, setting, and participants: This secondary analysis of an open-label noninferiority randomized clinical trial was a multicenter study conducted at 5 US academic medical centers from April 7, 2017, to November 11, 2022. Analyses for this study, which were not prespecified in the trial protocol, were conducted from May 10 to Oct 31, 2023. The study cohort included patients with TRD, aged 21 to 75 years, who were in a current nonpsychotic depressive episode of at least moderate severity and were referred for ECT by their clinicians.
Exposures: Eligible participants were randomized 1:1 to receive either 6 infusions of ketamine or 9 treatments with ECT over 3 weeks.
Main outcomes and measures: Association between baseline factors (including 16-item Quick Inventory of Depressive Symptomatology Self-Report [QIDS-SR16], Montgomery-Asberg Depression Rating Scale [MADRS], premorbid intelligence, cognitive function, history of attempted suicide, and inpatient vs outpatient status) and treatment response were assessed with repeated measures mixed-effects model analyses.
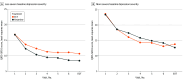
Results: Among the 365 participants included in this study (mean [SD] age, 46.0 [14.5] years; 191 [52.3%] female), 195 were randomized to the ketamine group and 170 to the ECT group. In repeated measures mixed-effects models using depression levels over 3 weeks and after false discovery rate adjustment, participants with a baseline QIDS-SR16 score of 20 or less (-7.7 vs -5.6 points) and those starting treatment as outpatients (-8.4 vs -6.2 points) reported greater reduction in the QIDS-SR16 with ketamine vs ECT. Conversely, those with a baseline QIDS-SR16 score of more than 20 (ie, very severe depression) and starting treatment as inpatients reported greater reduction in the QIDS-SR16 earlier in course of treatment (-8.4 vs -6.7 points) with ECT, but scores were similar in both groups at the end-of-treatment visit (-9.0 vs -9.9 points). In the ECT group only, participants with higher scores on measures of premorbid intelligence (-14.0 vs -11.2 points) and with a comorbid posttraumatic stress disorder diagnosis (-16.6 vs -12.0 points) reported greater reduction in the MADRS score. Those with impaired memory recall had greater reduction in MADRS during the second week of treatment (-13.4 vs -9.6 points), but the levels of MADRS were similar to those with unimpaired recall at the end-of-treatment visit (-14.3 vs -12.2 points). Other results were not significant after false discovery rate adjustment.
Conclusions and relevance: In this secondary analysis of the ELEKT-D randomized clinical trial of ECT vs ketamine, greater improvement in depression was observed with intravenous ketamine among outpatients with nonpsychotic TRD who had moderately severe or severe depression, suggesting that these patients may consider ketamine over ECT for TRD.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

