Targeting DRP1 with Mdivi-1 to correct mitochondrial abnormalities in ADOA+ syndrome
- PMID: 38916953
- PMCID: PMC11383607
- DOI: 10.1172/jci.insight.180582
Targeting DRP1 with Mdivi-1 to correct mitochondrial abnormalities in ADOA+ syndrome
Abstract
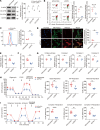
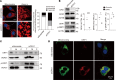
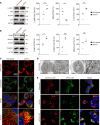
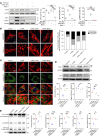
Autosomal dominant optic atrophy plus (ADOA+) is characterized by primary optic nerve atrophy accompanied by a spectrum of degenerative neurological symptoms. Despite ongoing research, no effective treatments are currently available for this condition. Our study provided evidence for the pathogenicity of an unreported c.1780T>C variant in the OPA1 gene through patient-derived skin fibroblasts and an engineered HEK293T cell line with OPA1 downregulation. We demonstrate that OPA1 insufficiency promoted mitochondrial fragmentation and increased DRP1 expression, disrupting mitochondrial dynamics. Consequently, this disruption enhanced mitophagy and caused mitochondrial dysfunction, contributing to the ADOA+ phenotype. Notably, the Drp1 inhibitor, mitochondrial division inhibitor-1 (Mdivi-1), effectively mitigated the adverse effects of OPA1 impairment. These effects included reduced Drp1 phosphorylation, decreased mitochondrial fragmentation, and balanced mitophagy. Thus, we propose that intervening in DRP1 with Mdivi-1 could correct mitochondrial abnormalities, offering a promising therapeutic approach for managing ADOA+.
Keywords: Autophagy; Drug therapy; Mitochondria; Neuroscience; Ophthalmology.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

