SLC44A2 regulates vascular smooth muscle cell phenotypic switching and aortic aneurysm
- PMID: 38916960
- PMCID: PMC11324303
- DOI: 10.1172/JCI173690
SLC44A2 regulates vascular smooth muscle cell phenotypic switching and aortic aneurysm
Abstract
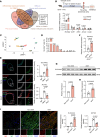
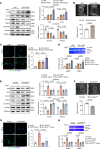
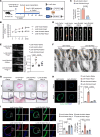
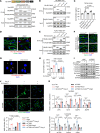
Aortic aneurysm is a life-threatening disease with limited interventions that is closely related to vascular smooth muscle cell (VSMC) phenotypic switching. SLC44A2, a member of the solute carrier series 44 (SLC44) family, remains undercharacterized in the context of cardiovascular diseases. Venn diagram analysis based on microarray and single-cell RNA sequencing identified SLC44A2 as a major regulator of VSMC phenotypic switching in aortic aneurysm. Screening for Slc44a2 among aortic cell lineages demonstrated its predominant location in VSMCs. Elevated levels of SLC44A2 were evident in the aorta of both patients with abdominal aortic aneurysm and angiotensin II-infused (Ang II-infused) Apoe-/- mice. In vitro, SLC44A2 silencing promoted VSMCs toward a synthetic phenotype, while SLC44A2 overexpression attenuated VSMC phenotypic switching. VSMC-specific SLC44A2-knockout mice were more susceptible to aortic aneurysm under Ang II infusion, while SLC44A2 overexpression showed protective effects. Mechanistically, SLC44A2's interaction with NRP1 and ITGB3 activates TGF-β/SMAD signaling, thereby promoting contractile gene expression. Elevated SLC44A2 in aortic aneurysm is associated with upregulated runt-related transcription factor 1 (RUNX1). Furthermore, low-dose lenalidomide (LEN; 20 mg/kg/day) suppressed aortic aneurysm progression by enhancing SLC44A2 expression. These findings reveal that the SLC44A2-NRP1-ITGB3 complex is a major regulator of VSMC phenotypic switching and provide a potential therapeutic approach (LEN) for aortic aneurysm treatment.
Keywords: Cardiovascular disease; Signal transduction; Vascular biology.
Figures




Comment in
- SLC44A2-mediated phenotypic switch of vascular smooth muscle cells contributes to aortic aneurysm
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

