Parvalbumin gates chronic pain through the modulation of firing patterns in inhibitory neurons
- PMID: 38916998
- PMCID: PMC11228497
- DOI: 10.1073/pnas.2403777121
Parvalbumin gates chronic pain through the modulation of firing patterns in inhibitory neurons
Abstract
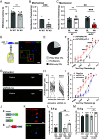
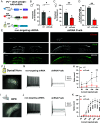
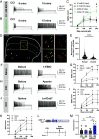
Spinal cord dorsal horn inhibition is critical to the processing of sensory inputs, and its impairment leads to mechanical allodynia. How this decreased inhibition occurs and whether its restoration alleviates allodynic pain are poorly understood. Here, we show that a critical step in the loss of inhibitory tone is the change in the firing pattern of inhibitory parvalbumin (PV)-expressing neurons (PVNs). Our results show that PV, a calcium-binding protein, controls the firing activity of PVNs by enabling them to sustain high-frequency tonic firing patterns. Upon nerve injury, PVNs transition to adaptive firing and decrease their PV expression. Interestingly, decreased PV is necessary and sufficient for the development of mechanical allodynia and the transition of PVNs to adaptive firing. This transition of the firing pattern is due to the recruitment of calcium-activated potassium (SK) channels, and blocking them during chronic pain restores normal tonic firing and alleviates chronic pain. Our findings indicate that PV is essential for controlling the firing pattern of PVNs and for preventing allodynia. Developing approaches to manipulate these mechanisms may lead to different strategies for chronic pain relief.
Keywords: chronic pain; dorsal horn; parvalbumin.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Jensen M. P., Chodroff M. J., Dworkin R. H., The impact of neuropathic pain on health-related quality of life: Review and implications. Neurology 68, 1178–1182 (2007). - PubMed
-
- Castro-Lopes J. M., Tavares I., Coimbra A., GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 620, 287–291 (1993). - PubMed
-
- Coull J. A. M., et al. , Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

