TRPV4 neuromuscular disease registry highlights bulbar, skeletal and proximal limb manifestations
- PMID: 38917025
- PMCID: PMC12054732
- DOI: 10.1093/brain/awae201
TRPV4 neuromuscular disease registry highlights bulbar, skeletal and proximal limb manifestations
Abstract
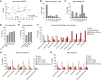
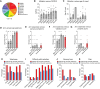
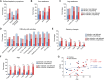
Dominant missense mutations of the calcium-permeable cation channel TRPV4 cause Charcot-Marie-Tooth disease (CMT) type 2C and two forms of distal spinal muscular atrophy. These conditions are collectively referred to as TRPV4-related neuromuscular disease and share features of motor greater than sensory dysfunction and frequent vocal fold weakness. Pathogenic variants lead to gain of ion channel function that can be rescued by TRPV4 antagonists in cellular and animal models. As small molecule TRPV4 antagonists have proven safe in trials for other disease indications, channel inhibition is a promising therapeutic strategy for TRPV4 patients. However, the current knowledge of the clinical features and natural history of TRPV4-related neuromuscular disease is insufficient to enable rational clinical trial design. To address these issues, we developed a TRPV4 patient database and administered a TRPV4-specific patient questionnaire. Here, we report demographic and clinical information, including CMT Examination Scores (CMTES), from 68 patients with known pathogenic TRPV4 variants, 40 of whom also completed the TRPV4 patient questionnaire. TRPV4 patients showed a bimodal age of onset, with the largest peak occurring in the first 2 years of life. Compared to CMT type 1A (CMT1A) patients, TRPV4 patients showed distinct symptoms and signs, manifesting more ambulatory difficulties and more frequent involvement of proximal arm and leg muscles. Although patients reported fewer sensory symptoms, sensory dysfunction was often detected clinically. Many patients were affected by vocal fold weakness (55%) and shortness of breath (55%), and 11% required ventilatory support. Skeletal abnormalities were common, including scoliosis (64%), arthrogryposis (33%) and foot deformities. Strikingly, patients with infantile onset of disease showed less sensory involvement and less progression of symptoms. These results highlight distinctive clinical features in TRPV4 patients, including motor-predominant disease, proximal arm and leg weakness, severe ambulatory difficulties, vocal fold weakness, respiratory dysfunction and skeletal involvement. In addition, patients with infantile onset of disease appeared to have a distinct phenotype with less apparent disease progression based on CMTES. These collective observations indicate that clinical trial design for TRPV4-related neuromuscular disease should include outcome measures that reliably capture non-length dependent motor dysfunction, vocal fold weakness and respiratory disease.
Keywords: CMT2C; Charcot-Marie-Tooth disease; TRPV4; hereditary neuropathy; spinal muscular atrophy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
M.H., S.T., T.O.C., C.J.S and B.A.M. are participating in an investigator-initiated natural history study for TRPV4 neuropathy (NCT05600764) that is supported by Actio Biosciences. C.S. is supported by Actio Biosciences for laboratory-based studies of TRPV4 channelopathy. D.N.H. has served as a consultant for Regenacy, Applied Therapeutics, DTx Pharma, Passage Bio, Roche, Pfizer, Orthogonal Neurosciences, NMD Pharma, Sarepta, GLG, Guidepoint Global. D.P. acknowledges payments or reimbursements for consultancy and participation in Advisory Boards of Inflectis, Augustine Tx, and DTx Pharma and participation as site PI in clinical trial sponsored by AT Therapeutics.
Figures



References
-
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet. 1974;6:98–118. - PubMed
-
- Barreto LCLS, Oliveira FS, Nunes PS, et al. Epidemiologic study of Charcot-Marie-Tooth disease: A systematic review. Neuroepidemiology. 2016;46:157–165. - PubMed
-
- Pareyson D, Marchesi C, Salsano E. Dominant Charcot-Marie-Tooth syndrome and cognate disorders. Handb Clin Neurol. 2013;115:817–845. - PubMed
-
- Laura M, Pipis M, Rossor AM, Reilly MM. Charcot-Marie-Tooth disease and related disorders: An evolving landscape. Curr Opin Neurol. 2019;32:641–650. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

