Development of a Potent and Selective G2A (GPR132) Agonist
- PMID: 38917049
- PMCID: PMC11249017
- DOI: 10.1021/acs.jmedchem.3c02164
Development of a Potent and Selective G2A (GPR132) Agonist
Abstract
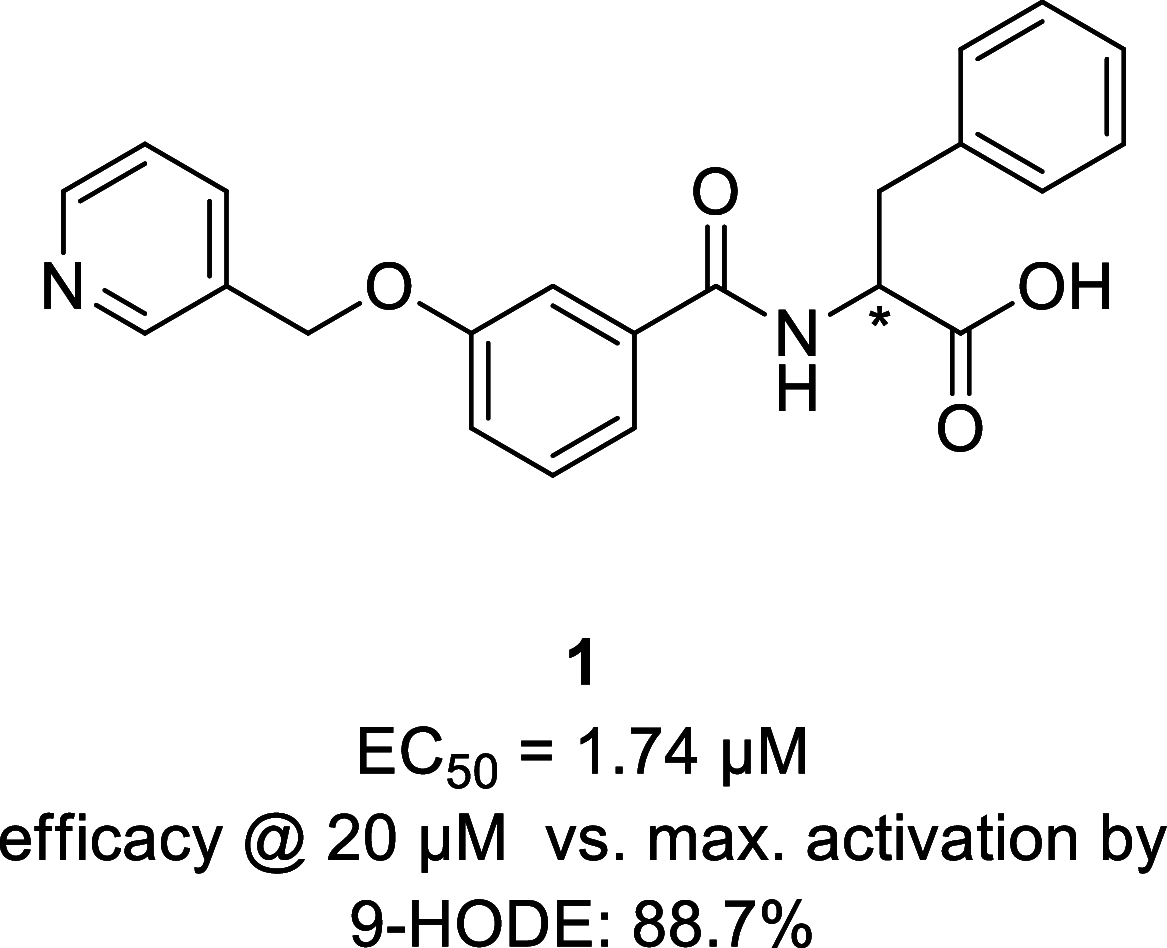
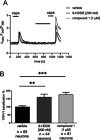
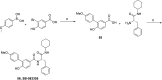
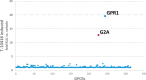
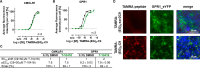
G protein-coupled receptor G2A was postulated to be a promising target for the development of new therapeutics in neuropathic pain, acute myeloid leukemia, and inflammation. However, there is still a lack of potent, selective, and drug-like G2A agonists to be used as a chemical tool or as the starting matter for the development of drugs. In this work, we present the discovery and structure-activity relationship elucidation of a new potent and selective G2A agonist scaffold. Systematic optimization resulted in (3-(pyridin-3-ylmethoxy)benzoyl)-d-phenylalanine (T-10418) exhibiting higher potency than the reference and natural ligand 9-HODE and high selectivity among G protein-coupled receptors. With its favorable activity, a clean selectivity profile, excellent solubility, and high metabolic stability, T-10418 qualifies as a pharmacological tool to investigate the effects of G2A activation.
Conflict of interest statement
The authors declare the following competing financial interest(s): Y.S.M. is a CEO at Chemspace LLC and a scientific advisor at Enamine Ltd. V.V.I. is a Chief Sales Officer at Enamine Ltd.
Figures


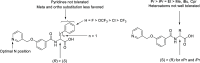




References
-
- Weng Z.; Fluckiger A.-C.; Nisitani S.; Wahl M. I.; Le L. Q.; Hunter C. A.; Fernal A. A.; Le Beau M. M.; Witte O. N. A DNA Damage and Stress Inducible G Protein-Coupled Receptor Blocks Cells in G 2/M. Proc. Natl. Acad. Sci. U.S.A. 1998, 95 (21), 12334–12339. 10.1073/pnas.95.21.12334. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

