Association of Levels of CSF Osteopontin With Cortical Atrophy and Disability in Early Multiple Sclerosis
- PMID: 38917380
- PMCID: PMC11203401
- DOI: 10.1212/NXI.0000000000200265
Association of Levels of CSF Osteopontin With Cortical Atrophy and Disability in Early Multiple Sclerosis
Abstract
Background and objectives: To evaluate CSF inflammatory markers with accumulation of cortical damage as well as disease activity in patients with early relapsing-remitting MS (RRMS).
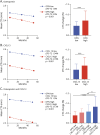
Methods: CSF levels of osteopontin (OPN) and 66 inflammatory markers were assessed using an immune-assay multiplex technique in 107 patients with RRMS (82 F/25 M, mean age 35.7 ± 11.8 years). All patients underwent regular clinical assessment and yearly 3T MRI scans for 2 years while 39 patients had a 4-year follow-up. White matter lesion number and volume, cortical lesions (CLs) and volume, and global cortical thickness (CTh) were evaluated together with the 'no evidence of disease activity' (NEDA-3) status, defined by no relapses, no disability worsening, and no MRI activity, including CLs.
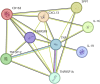
Results: The random forest algorithm selected OPN, CXCL13, TWEAK, TNF, IL19, sCD30, sTNFR1, IL35, IL16, and sCD163 as significantly associated with changes in global CTh. OPN and CXCL13 were most related to accumulation of atrophy after 2 and 4 years. In a multivariate linear regression model on CSF markers, OPN (p < 0.001), CXCL13 (p = 0.001), and sTNFR1 (p = 0.024) were increased in those patients with accumulating atrophy (adjusted R-squared 0.615). The 10 markers were added in a model that included all clinical, demographic, and MRI variables: OPN (p = 0.002) and IL19 (p = 0.022) levels were confirmed to be significantly increased in patients developing more CTh change over the follow-up (adjusted R-squared 0.619). CXCL13 and OPN also revealed the best association with NEDA-3 after 2 years, with OPN significantly linked to disability accumulation (OR 2.468 [1.46-5.034], p = 0.004) at the multivariate logistic regression model.
Discussion: These data confirm and expand our knowledge on the prognostic role of the CSF inflammatory profile in predicting changes in cortical pathology and disease activity in early MS. The data emphasize a crucial role of OPN.
Conflict of interest statement
M. Calabrese was supported by the GR-2013-02-355322 grant from the Italian Ministry of Health. Go to
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
