N-terminal cysteine acetylation and oxidation patterns may define protein stability
- PMID: 38918375
- PMCID: PMC11199558
- DOI: 10.1038/s41467-024-49489-2
N-terminal cysteine acetylation and oxidation patterns may define protein stability
Abstract
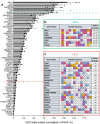
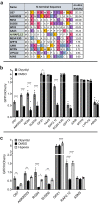
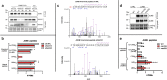
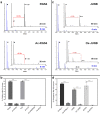
Oxygen homeostasis is maintained in plants and animals by O2-sensing enzymes initiating adaptive responses to low O2 (hypoxia). Recently, the O2-sensitive enzyme ADO was shown to initiate degradation of target proteins RGS4/5 and IL32 via the Cysteine/Arginine N-degron pathway. ADO functions by catalysing oxidation of N-terminal cysteine residues, but despite multiple proteins in the human proteome having an N-terminal cysteine, other endogenous ADO substrates have not yet been identified. This could be because alternative modifications of N-terminal cysteine residues, including acetylation, prevent ADO-catalysed oxidation. Here we investigate the relationship between ADO-catalysed oxidation and NatA-catalysed acetylation of a broad range of protein sequences with N-terminal cysteines. We present evidence that human NatA catalyses N-terminal cysteine acetylation in vitro and in vivo. We then show that sequences downstream of the N-terminal cysteine dictate whether this residue is oxidised or acetylated, with ADO preferring basic and aromatic amino acids and NatA preferring acidic or polar residues. In vitro, the two modifications appear to be mutually exclusive, suggesting that distinct pools of N-terminal cysteine proteins may be acetylated or oxidised. These results reveal the sequence determinants that contribute to N-terminal cysteine protein modifications, with implications for O2-dependent protein stability and the hypoxic response.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

