Efficient and highly reproducible production of red blood cell-derived extracellular vesicle mimetics for the loading and delivery of RNA molecules
- PMID: 38918594
- PMCID: PMC11199497
- DOI: 10.1038/s41598-024-65623-y
Efficient and highly reproducible production of red blood cell-derived extracellular vesicle mimetics for the loading and delivery of RNA molecules
Abstract
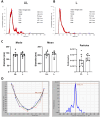
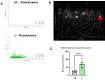
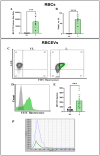
Extracellular vesicles (EVs) are promising natural nanocarriers for the delivery of therapeutic agents. As with any other kind of cell, red blood cells (RBCs) produce a limited number of EVs under physiological and pathological conditions. Thus, RBC-derived extracellular vesicles (RBCEVs) have been recently suggested as next-generation delivery systems for therapeutic purposes. In this paper, we show that thanks to their unique biological and physicochemical features, RBCs can be efficiently pre-loaded with several kinds of molecules and further used to generate RBCEVs. A physical vesiculation method, based on "soft extrusion", was developed, producing an extremely high yield of cargo-loaded RBCEV mimetics. The RBCEVs population has been deeply characterized according to the new guidelines MISEV2023, showing great homogeneity in terms of size, biological features, membrane architecture and cargo. In vitro preliminary results demonstrated that RBCEVs are abundantly internalized by cells and exert peculiar biological effects. Indeed, efficient loading and delivery of miR-210 by RBCEVs to HUVEC has been proven, as well as the inhibition of a known mRNA target. Of note, the bench-scale process can be scaled-up and translated into clinics. In conclusion, this investigation could open the way to a new biomimetic platform for RNA-based therapies and/or other therapeutic cargoes useful in several diseases.
Keywords: Drug delivery; EV engineering; Extracellular vesicles; RBC-derived extracellular vesicles; RNA therapy; miRNAs.
© 2024. The Author(s).
Conflict of interest statement
All the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors also declare that some of the data presented herein have been used to fill a patent application (patent application number 102023000026244).
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

