Evaluating the immunologically "cold" tumor microenvironment after treatment with immune checkpoint inhibitors utilizing PET imaging of CD4 + and CD8 + T cells in breast cancer mouse models
- PMID: 38918836
- PMCID: PMC11201779
- DOI: 10.1186/s13058-024-01844-3
Evaluating the immunologically "cold" tumor microenvironment after treatment with immune checkpoint inhibitors utilizing PET imaging of CD4 + and CD8 + T cells in breast cancer mouse models
Abstract
Background: Immune-positron emission tomography (PET) imaging with tracers that target CD8 and granzyme B has shown promise in predicting the therapeutic response following immune checkpoint blockade (ICB) in immunologically "hot" tumors. However, immune dynamics in the low T-cell infiltrating "cold" tumor immune microenvironment during ICB remain poorly understood. This study uses molecular imaging to evaluate changes in CD4 + T cells and CD8 + T cells during ICB in breast cancer models and examines biomarkers of response.
Methods: [89Zr]Zr-DFO-CD4 and [89Zr]Zr-DFO-CD8 radiotracers were used to quantify changes in intratumoral and splenic CD4 T cells and CD8 T cells in response to ICB treatment in 4T1 and MMTV-HER2 mouse models, which represent immunologically "cold" tumors. A correlation between PET quantification metrics and long-term anti-tumor response was observed. Further biological validation was obtained by autoradiography and immunofluorescence.
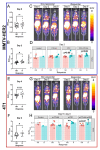
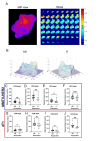
Results: Following ICB treatment, an increase in the CD8-specific PET signal was observed within 6 days, and an increase in the CD4-specific PET signal was observed within 2 days in tumors that eventually responded to immunotherapy, while no significant differences in CD4 or CD8 were found at the baseline of treatment that differentiated responders from nonresponders. Furthermore, mice whose tumors responded to ICB had a lower CD8 PET signal in the spleen and a higher CD4 PET signal in the spleen compared to non-responders. Intratumoral spatial heterogeneity of the CD8 and CD4-specific PET signals was lower in responders compared to non-responders. Finally, PET imaging, autoradiography, and immunofluorescence signals were correlated when comparing in vivo imaging to ex vivo validations.
Conclusions: CD4- and CD8-specific immuno-PET imaging can be used to characterize the in vivo distribution of CD4 + and CD8 + T cells in response to immune checkpoint blockade. Imaging metrics that describe the overall levels and distribution of CD8 + T cells and CD4 + T cells can provide insight into immunological alterations, predict biomarkers of response to immunotherapy, and guide clinical decision-making in those tumors where the kinetics of the response differ.
Keywords: 89Zr; 4T1; CTLA4; ImmunoPET; MMTV-HER2; PD-1; Positron emission tomography; Spatial heterogeneity; Spleen.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Hanahan D. Hallmarks of Cancer: New dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

