Synthetic applications of the Cannizzaro reaction
- PMID: 38919603
- PMCID: PMC11196959
- DOI: 10.3762/bjoc.20.120
Synthetic applications of the Cannizzaro reaction
Abstract
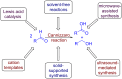
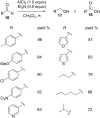
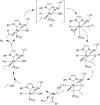
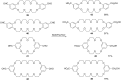
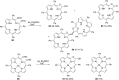
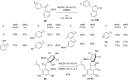
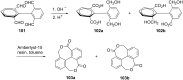
The Cannizzaro reaction has emerged as a versatile synthetic tool for the construction of functionalized molecules. Dating back to the 19th century, this reaction, though initially used for the synthesis of an alcohol and acid functionality from aldehydes, has henceforth proven useful to generate diverse molecular entities using both intermolecular and intramolecular synthetic strategies. Immense applications in the synthesis of hydroxy acids and esters, heterocycles, fused carbocycles, natural products, and others with broad substrate scope have raised profound interest from methodological and synthetic standpoints. The ongoing development of reagents, solvents, and technologies for the Cannizzaro reaction reflects the broader trend in organic synthesis towards more sustainable and efficient practices. The focus of this review is to highlight some recent advances in synthetic strategies and applications of the Cannizzaro reaction towards the synthesis of potentially useful molecules.
Keywords: Cannizzaro reaction; Lewis acid catalyst; crossed-Cannizzaro; desymmetrization; natural products.
Copyright © 2024, Chatterjee et al.
Figures

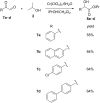
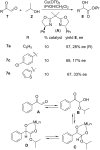
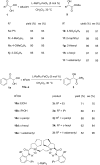

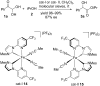





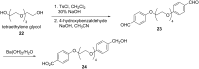






References
-
- Cannizzaro S. Justus Liebigs Ann Chem. 1853;88:129–130. doi: 10.1002/jlac.18530880114. - DOI
-
- Geissman T A. Org React. 1944;2:94–113. doi: 10.1002/0471264180.or002.03. - DOI
-
- Fry E M, Wilson E J, Jr, Hudson C S. J Am Chem Soc. 1942;64:872–873. doi: 10.1021/ja01256a038. - DOI
-
- Chandrasekhar S, Srimannarayana M. Synth Commun. 2009;39:4473–4478. doi: 10.1080/00397910902916049. - DOI
Publication types
LinkOut - more resources
Full Text Sources
