Partial hepatectomy accelerates colorectal metastasis by priming an inflammatory premetastatic niche in the liver
- PMID: 38919609
- PMCID: PMC11196966
- DOI: 10.3389/fimmu.2024.1388272
Partial hepatectomy accelerates colorectal metastasis by priming an inflammatory premetastatic niche in the liver
Abstract
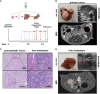
Background: Resection of colorectal liver metastasis is the standard of care for patients with Stage IV CRC. Despite undoubtedly improving the overall survival of patients, pHx for colorectal liver metastasis frequently leads to disease recurrence. The contribution of this procedure to metastatic colorectal cancer at a molecular level is poorly understood. We designed a mouse model of orthograde metastatic colorectal cancer (CRC) to investigate the effect of partial hepatectomy (pHx) on tumor progression.
Methods: CRC organoids were implanted into the cecal walls of wild type mice, and animals were screened for liver metastasis. At the time of metastasis, 1/3 partial hepatectomy was performed and the tumor burden was assessed longitudinally using MRI. After euthanasia, different tissues were analyzed for immunological and transcriptional changes using FACS, qPCR, RNA sequencing, and immunohistochemistry.
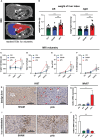
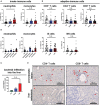
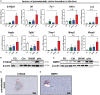
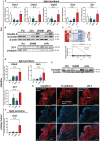
Results: Mice that underwent pHx presented significant liver hypertrophy and an increased overall metastatic load compared with SHAM operated mice in MRI. Elevation in the metastatic volume was defined by an increase in de novo liver metastasis without any effect on the growth of each metastasis. Concordantly, the livers of pHx mice were characterized by neutrophil and bacterial infiltration, inflammatory response, extracellular remodeling, and an increased abundance of tight junctions, resulting in the formation of a premetastatic niche, thus facilitating metastatic seeding.
Conclusions: Regenerative pathways following pHx accelerate colorectal metastasis to the liver by priming a premetastatic niche.
Keywords: colorectal cancer; liver metastasis; partial hepatectomy; premetastatic niche; tight junctions.
Copyright © 2024 Luenstedt, Hoping, Feuerstein, Mauerer, Berlin, Rapp, Marx, Reichardt, von Elverfeldt, Ruess, Plundrich, Laessle, Jud, Neeff, Holzner, Fichtner-Feigl and Kesselring.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. . High survival rate after two-stage resection of advanced colorectal liver metastases: Response-based selection and complete resection define outcome. J Clin Oncol. (2011) 29:1083–90. doi: 10.1200/JCO.2010.32.6132 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

