Elucidating the molecular landscape of tendinitis: the role of inflammasome-related genes and immune interactions
- PMID: 38919626
- PMCID: PMC11196777
- DOI: 10.3389/fimmu.2024.1393851
Elucidating the molecular landscape of tendinitis: the role of inflammasome-related genes and immune interactions
Abstract
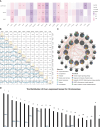
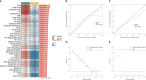
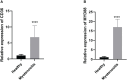
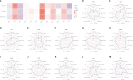
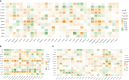
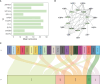
Tendinitis, characterized by the inflammation of tendons, poses significant challenges in both diagnosis and treatment due to its multifaceted etiology and complex pathophysiology. This study aimed to dissect the molecular mechanisms underlying tendinitis, with a particular focus on inflammasome-related genes and their interactions with the immune system. Through comprehensive gene expression analysis and bioinformatics approaches, we identified distinct expression profiles of inflammasome genes, such as NLRP6, NLRP1, and MEFV, which showed significant correlations with immune checkpoint molecules, indicating a pivotal role in the inflammatory cascade of tendinitis. Additionally, MYD88 and CD36 were found to be closely associated with HLA family molecules, underscoring their involvement in immune response modulation. Contrary to expectations, chemokines exhibited minimal correlation with inflammasome genes, suggesting an unconventional inflammatory pathway in tendinitis. Transcription factors like SP110 and CREB5 emerged as key regulators of inflammasome genes, providing insight into the transcriptional control mechanisms in tendinitis. Furthermore, potential therapeutic targets were identified through the DGidb database, highlighting drugs that could modulate the activity of inflammasome genes, offering new avenues for targeted tendinitis therapy. Our findings elucidate the complex molecular landscape of tendinitis, emphasizing the significant role of inflammasomes and immune interactions, and pave the way for the development of novel diagnostic and therapeutic strategies.
Keywords: bioinformatics; gene expression; immune system; inflammasomes; tendinitis.
Copyright © 2024 Xu, Lu, Yu, Zhou, Qi and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Expression profiles of NOD-like receptors and regulation of NLRP3 inflammasome activation in Toxoplasma gondii-infected human small intestinal epithelial cells.Parasit Vectors. 2021 Mar 12;14(1):153. doi: 10.1186/s13071-021-04666-w. Parasit Vectors. 2021. PMID: 33712075 Free PMC article.
-
Cyclic Stretching Exacerbates Tendinitis by Enhancing NLRP3 Inflammasome Activity via F-Actin Depolymerization.Inflammation. 2018 Oct;41(5):1731-1743. doi: 10.1007/s10753-018-0816-5. Inflammation. 2018. PMID: 29951874
-
Production of IL-1β and Inflammasome with Up-Regulated Expressions of NOD-Like Receptor Related Genes in Toxoplasma gondii-Infected THP-1 Macrophages.Korean J Parasitol. 2016 Dec;54(6):711-717. doi: 10.3347/kjp.2016.54.6.711. Epub 2016 Dec 31. Korean J Parasitol. 2016. PMID: 28095655 Free PMC article.
-
Inflammasomes on the Crossroads of Innate Immune Recognition and Metabolic Control.Cell Metab. 2017 Jul 5;26(1):71-93. doi: 10.1016/j.cmet.2017.06.018. Cell Metab. 2017. PMID: 28683296 Review.
-
Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
References
-
- Churgay CA. Diagnosis and treatment of biceps tendinitis and tendinosis. Am Family physician. (2009) 80:470–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

