Mechanism, structural and functional insights into nidovirus-induced double-membrane vesicles
- PMID: 38919631
- PMCID: PMC11196420
- DOI: 10.3389/fimmu.2024.1340332
Mechanism, structural and functional insights into nidovirus-induced double-membrane vesicles
Abstract
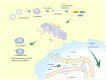
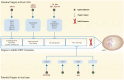
During infection, positive-stranded RNA causes a rearrangement of the host cell membrane, resulting in specialized membrane structure formation aiding viral genome replication. Double-membrane vesicles (DMVs), typical structures produced by virus-induced membrane rearrangements, are platforms for viral replication. Nidoviruses, one of the most complex positive-strand RNA viruses, have the ability to infect not only mammals and a few birds but also invertebrates. Nidoviruses possess a distinctive replication mechanism, wherein their nonstructural proteins (nsps) play a crucial role in DMV biogenesis. With the participation of host factors related to autophagy and lipid synthesis pathways, several viral nsps hijack the membrane rearrangement process of host endoplasmic reticulum (ER), Golgi apparatus, and other organelles to induce DMV formation. An understanding of the mechanisms of DMV formation and its structure and function in the infectious cycle of nidovirus may be essential for the development of new and effective antiviral strategies in the future.
Keywords: DMV; membrane remodeling; nidoviruses; virus replication; virus-cell interaction.
Copyright © 2024 Wang, Chen, Qi, Li, Zhang, Zhou, Wu, Zhang, Qi, Ouyang, Xie and Pang.
Conflict of interest statement
Authors HO and DP were employed by the company Chongqing Jitang Biotechnology Research Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Expression and Cleavage of Middle East Respiratory Syndrome Coronavirus nsp3-4 Polyprotein Induce the Formation of Double-Membrane Vesicles That Mimic Those Associated with Coronaviral RNA Replication.mBio. 2017 Nov 21;8(6):e01658-17. doi: 10.1128/mBio.01658-17. mBio. 2017. PMID: 29162711 Free PMC article.
-
Biogenesis and architecture of arterivirus replication organelles.Virus Res. 2016 Jul 15;220:70-90. doi: 10.1016/j.virusres.2016.04.001. Epub 2016 Apr 9. Virus Res. 2016. PMID: 27071852 Free PMC article. Review.
-
The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses.Cell Mol Life Sci. 2022 Jul 16;79(8):425. doi: 10.1007/s00018-022-04469-x. Cell Mol Life Sci. 2022. PMID: 35841484 Free PMC article. Review.
-
Mutations across murine hepatitis virus nsp4 alter virus fitness and membrane modifications.J Virol. 2015 Feb;89(4):2080-9. doi: 10.1128/JVI.02776-14. Epub 2014 Dec 3. J Virol. 2015. PMID: 25473044 Free PMC article.
-
Antiviral Innate Immune Response Interferes with the Formation of Replication-Associated Membrane Structures Induced by a Positive-Strand RNA Virus.mBio. 2016 Dec 6;7(6):e01991-16. doi: 10.1128/mBio.01991-16. mBio. 2016. PMID: 27923923 Free PMC article.
References
-
- Bryans JT, Crowe ME, Doll ER, McCollum WH. Isolation of a filterable agent causing arteritis of horses and abortion by mares; its differentiation from the equine abortion (influenza) virus. Cornell Vet. (1957) 47:3–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

