Nicotine and fluoxetine alter adolescent dopamine-mediated behaviors via 5-HT1A receptor activation
- PMID: 38919632
- PMCID: PMC11196788
- DOI: 10.3389/fpsyt.2024.1380123
Nicotine and fluoxetine alter adolescent dopamine-mediated behaviors via 5-HT1A receptor activation
Abstract
Introduction: Abuse or misuse of tobacco, e-cigarettes, or antidepressants may have serious clinical consequences during adolescence, a sensitive period during brain development when the distinct neurobiology of adolescent serotonin (5-HT) and dopamine (DA) systems create unique behavioral vulnerabilities to drugs of abuse.
Methods: Using a pharmacological approach, we modeled the behavioral and neurochemical effects of subchronic (4-day) nicotine (60µg/kg, i.v.) or fluoxetine (1mg/kg, i.v.) exposure in adolescent and adult male rats.
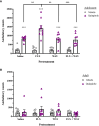
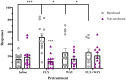
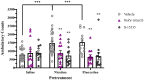
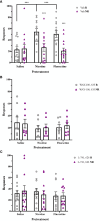
Results: Nicotine and fluoxetine significantly enhance quinpirole-induced locomotor activity and initial cocaine self-administration in adolescents, but not adults. These effects were blocked by serotonin 5-HT1A receptor antagonists, WAY-100,635 (100 µg/kg, i.v.) or S-15535 (300 µg/kg, i.v.). Neurochemical and anatomical autoradiographic analysis of 8-OH-DPAT-stimulated [35S]GTPγS reveal that prior exposure to nicotine and fluoxetine results in both overlapping and distinct effects on regional 5-HT1A receptor activity. Both fluoxetine and nicotine enhance adolescent 5-HT1A receptor activity in the primary motor cortex (M1), whereas fluoxetine alone targets prefrontal cortical neurocircuitry and nicotine alone targets the amygdala.
Discussion: Given their different pharmacological profiles, comparison between WAY-100,635 and S-15535 indicates that postsynaptic 5-HT1A receptors mediate the behavioral effects of prior nicotine and fluoxetine exposure. In addition, within the adolescent M1, maladaptive changes in 5-HT signaling and 5-HT1A activity after nicotine or fluoxetine exposure may potentiate hyper-responsiveness to dopaminergic drugs and prime adolescent vulnerability for future substance abuse.
Keywords: adolescence; age-dependent; behavioral acquisition; cocaine; fluoxetine; nicotine; self-administration.
Copyright © 2024 Yuan and Leslie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism.Neuropsychopharmacology. 2011 Jun;36(7):1319-31. doi: 10.1038/npp.2011.8. Epub 2011 Mar 16. Neuropsychopharmacology. 2011. PMID: 21412223 Free PMC article.
-
Chronic treatment with fluoxetine decreases cerebral metabolic responses to the 5-HT1A agonist 8-hydroxy-2(di-N-propylamino)tetralin and increases those to the 5-HT2A/2C agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane and to the dopaminergic agonist apomorphine.Brain Res. 2010 Jun 4;1335:24-34. doi: 10.1016/j.brainres.2010.03.090. Epub 2010 Apr 8. Brain Res. 2010. PMID: 20381465
-
WAY100635 prevents the changes induced by fluoxetine upon the 5-HT1A receptor functionality.Neuropharmacology. 2008 Dec;55(8):1391-6. doi: 10.1016/j.neuropharm.2008.08.038. Epub 2008 Sep 17. Neuropharmacology. 2008. PMID: 18809415
-
Effect of chronic fluoxetine and WAY-100635 treatment on serotonergic neurotransmission in the frontal cortex.J Psychopharmacol. 2002 Jun;16(2):145-52. doi: 10.1177/026988110201600205. J Psychopharmacol. 2002. PMID: 12095073
-
S 15535, a novel benzodioxopiperazine ligand of serotonin (5-HT)1A receptors: I. Interaction with cloned human (h)5-HT1A, dopamine hD2/hD3 and h alpha2A-adrenergic receptors in relation to modulation of cortical monoamine release and activity in models of potential antidepressant activity.J Pharmacol Exp Ther. 1997 Jul;282(1):132-47. J Pharmacol Exp Ther. 1997. PMID: 9223549
Cited by
-
Biotin Mitigates Alcohol Withdrawal-Induced Anxiety and Depression by Regulating Serotonin Metabolism, BDNF, Inflammation, and Oxidative Stress in Rats.Neuropsychopharmacol Rep. 2025 Mar;45(1):e12523. doi: 10.1002/npr2.12523. Neuropsychopharmacol Rep. 2025. PMID: 39754400 Free PMC article.
References
LinkOut - more resources
Full Text Sources

