Ravulizumab use for acetylcholine receptor-positive generalized myasthenia gravis in clinical practice
- PMID: 38919970
- PMCID: PMC11197931
- DOI: 10.3389/fneur.2024.1378080
Ravulizumab use for acetylcholine receptor-positive generalized myasthenia gravis in clinical practice
Abstract
Purpose: To describe the early experience of ravulizumab use in acetylcholine receptor antibody-positive generalized myasthenia gravis (AChR+ve gMG).
Methods: This multicenter retrospective study included AChR+ve gMG patients who were treated with ravulizumab and had both pre- and post-ravulizumab myasthenia gravis activities of daily living (MG-ADL) scores. Clinical information regarding MG history, concomitant treatment(s), MG-ADL, other MG-specific measures, and adverse events were recorded.
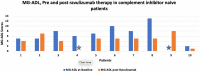
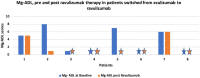
Results: A total of 18 patients with mean age of 61.83 (±16.08, n = 18) years were included in this cohort. In 10 complement inhibitor naive patients, a clinically meaningful reduction in mean Mg-ADL (baseline: 6.6 (±3.58) vs. 4.4 (±2.28), post ravulizumab) was seen. 6 out of 10 patients (60%) had clinically meaningful reduction post ravulizumab and two achieved minimum symptom expression (MSE). In 8 patients switched from eculizumab to ravulizumab, further reduction was noted in post ravulizumab mean MG-ADL (Baseline: 3.25 (±3.34) vs. 1.5 (±2.34) post ravulizumab). None of the patients who switched from eculizumab to ravulizumab experienced worsening symptoms. Eleven out of 14 (78.5%) patients on prednisone therapy were able to reduce their prednisone dose post-ravulizumab. None of the patients experienced any major side effects.
Conclusion: In our clinical practice, 60% of AChR+ve gMG complement inhibitor naive patients experienced a clinically meaningful improvement in MG-ADL scores with ravulizumab. Patients were safely switched from eculizumab to ravulizumab and had further improvement in their mean MG-ADL scores. Of those on prednisone therapy, the majority were able to reduce their prednisone dosage.
Keywords: MG-ADL; acetylcholine receptor antibody positive; complement inhibition; generalized myasthenia gravis; ravulizumab.
Copyright © 2024 Katyal, Govindarajan, Goyal, Muley and Muppidi.
Conflict of interest statement
RG served on the advisory board for Argenx, UCB, Janssen, Roche, and speakers bureau for Argenx and Alexion. NG has served on advisory board meetings for Argenx, Alexion, and UCB Pharma. SrM has served on advisory board meetings for Argenx, Alexion/Astrazaneca, ra/UCB, and Horizont Pharma. SuM speaker Bureau: Takeda, CSL Behring, Grifols, Catalyst, Alexion, Argenx. Consultant: UCB, Alexion, Argenx. Advisory Boards: Horizon, Argenx, Alexion, Grifols. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Howard JF, Jr, Bresch S, Genge A, Hewamadduma C, Hinton J, Hussain Y, et al. . Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. (2023) 22:395–406. doi: 10.1016/S1474-4422(23)00080-7, PMID: - DOI - PubMed
-
- Bril V, Drużdż A, Grosskreutz J, Habib AA, Mantegazza R, Sacconi S, et al. . Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. (2023) 22:383–94. doi: 10.1016/S1474-4422(23)00077-7, PMID: - DOI - PubMed
-
- Howard JF, Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. . Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

