Circadian-Dependent Intermittent Fasting Influences Ischemic Tolerance and Dendritic Spine Remodeling
- PMID: 38920050
- PMCID: PMC11262964
- DOI: 10.1161/STROKEAHA.124.046400
Circadian-Dependent Intermittent Fasting Influences Ischemic Tolerance and Dendritic Spine Remodeling
Abstract
Background: Preconditioning by intermittent fasting is linked to improved cognition and motor function, and enhanced recovery after stroke. Although the duration of fasting was shown to elicit different levels of neuroprotection after ischemic stroke, the impact of time of fasting with respect to the circadian cycles remains unexplored.
Methods: Cohorts of mice were subjected to a daily 16-hour fast, either during the dark phase (active-phase intermittent fasting) or the light phase (inactive-phase intermittent fasting) or were fed ad libitum. Following a 6-week dietary regimen, mice were subjected to transient focal cerebral ischemia and underwent behavioral functional assessment. Brain samples were collected for RNA sequencing and histopathologic analyses.
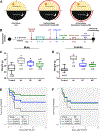
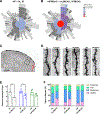
Results: Active-phase intermittent fasting cohort exhibited better poststroke motor and cognitive recovery as well as reduced infarction, in contrast to inactive-phase intermittent fasting cohort, when compared with ad libitum cohort. In addition, protection of dendritic spine density/morphology and increased expression of postsynaptic density protein-95 were observed in the active-phase intermittent fasting.
Conclusions: These findings indicate that the time of daily fasting is an important factor in inducing ischemic tolerance by intermittent fasting.
Keywords: cerebrovascular diseases; circadian rhythm; dendritic spines; intermittent fasting; postsynaptic density protein-95; structural plasticity.
Conflict of interest statement
None.
Figures




References
-
- St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2017;135:e96–e121. doi: 10.1161/cir.0000000000000476 - DOI - PMC - PubMed
-
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of neurochemistry. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x - DOI - PubMed
-
- Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordrecht, Netherlands). 2012;34:917–933. doi: 10.1007/s11357-011-9289-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

