Unveiling the Structural Properties, Optical Behavior, and Thermoelectric Performance of 2D CsSn2Br5 Halide Obtained by Mechanochemistry
- PMID: 38920333
- PMCID: PMC11234366
- DOI: 10.1021/acs.inorgchem.4c01861
Unveiling the Structural Properties, Optical Behavior, and Thermoelectric Performance of 2D CsSn2Br5 Halide Obtained by Mechanochemistry
Abstract
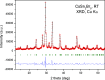
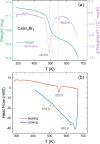
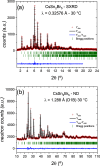
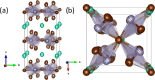
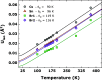
Metal halide perovskites with a two-dimensional structure are utilized in photovoltaics and optoelectronics. High-crystallinity CsSn2Br5 specimens have been synthesized via ball milling. Differential scanning calorimetry curves show melting at 553 K (endothermic) and recrystallization at 516 K (exothermic). Structural analysis using synchrotron X-ray diffraction data, collected from 100 to 373 K, allows for the determination of Debye model parameters. This analysis provides insights into the relative Cs-Br and Sn-Br chemical bonds within the tetragonal structure (space group: I4/mcm), which remains stable throughout the temperature range studied. Combined with neutron data, X-N techniques permit the identification of the Sn2+ lone electron pair (5s2) in the two-dimensional framework, occupying empty space opposite to the four Sn-Br bonds of the pyramidal [SnBr4] coordination polyhedra. Additionally, diffuse reflectance UV-vis spectroscopy unveils an indirect optical gap of approximately ∼3.3 eV, aligning with the calculated value from the B3LYP-DFT method (∼3.2 eV). The material exhibits a positive Seebeck coefficient as high as 6.5 × 104 μV K-1 at 350 K, which evolves down to negative values of -3.0 × 103 μV K-1 at 550 K, surpassing values reported for other halide perovskites. Notably, the thermal conductivity remains exceptionally low, between 0.32 and 0.25 W m-1 K-1.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Heo J. H.; Im S. H.; Noh J. H.; Mandal T. N.; Lim C.-S.; Chang J. A.; Lee Y. H.; Kim H.; Sarkar A.; Nazeeruddin M. K.; Grätzel M.; Seok S. Il. Efficient Inorganic–Organic Hybrid Heterojunction Solar Cells Containing Perovskite Compound and Polymeric Hole Conductors. Nat. Photonics 2013, 7 (6), 486–491. 10.1038/nphoton.2013.80. - DOI
-
- Li F.; Deng X.; Qi F.; Li Z.; Liu D.; Shen D.; Qin M.; Wu S.; Lin F.; Jang S.-H.; Zhang J.; Lu X.; Lei D.; Lee C.-S.; Zhu Z.; Jen A. K.-Y. Regulating Surface Termination for Efficient Inverted Perovskite Solar Cells with Greater Than 23% Efficiency. J. Am. Chem. Soc. 2020, 142 (47), 20134–20142. 10.1021/jacs.0c09845. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

