Last but not least: emerging roles of the autophagy-related protein ATG4D
- PMID: 38920354
- PMCID: PMC11346562
- DOI: 10.1080/15548627.2024.2369436
Last but not least: emerging roles of the autophagy-related protein ATG4D
Abstract
The evolutionarily conserved ATG4 cysteine proteases regulate macroautophagy/autophagy through the priming and deconjugation of the Atg8-family proteins. In mammals there are four ATG4 family members (ATG4A, ATG4B, ATG4C, ATG4D) but ATG4D has been relatively understudied. Heightened interest in ATG4D has been stimulated by recent links to human disease. Notably, genetic variations in human ATG4D were implicated in a heritable neurodevelopmental disorder. Genetic analyses in dogs, along with loss-of-function zebrafish and mouse models, further support a neuroprotective role for ATG4D. Here we discuss the evidence connecting ATG4D to neurological diseases and other pathologies and summarize its roles in both autophagy-dependent and autophagy-independent cellular processes.Abbrevation: ATG: autophagy related; BafA1: bafilomycin A1; BCL2: BCL2 apoptosis regulator; BH3: BCL2 homology region 3; CASP3: caspase 3; EV: extracellular vesicle; GABA: gamma aminobutyric acid; GABARAP: GABA type A receptor-associated protein; GABARAPL1: GABA type A receptor associated protein like 1; GABARAPL2: GABA type A receptor associated protein like 2; GFP: green fluorescent protein; LIR: LC3-interacting region; MAP1LC3: microtubule associated protein 1 light chain 3; MEF: mouse embryonic fibroblast; MYC: MYC proto-oncogene, bHLH transcription factor; PE: phosphatidylethanolamine; PS: phosphatidylserine; QKO: quadruple knockout; SDS-PAGE: sodium dodecyl sulfate-polyacrylamide gel; SQSTM1: sequestosome 1.
Keywords: Atg8; GABARAPL1; cancer; mitochondria; neurodegeneration; neurodevelopmental disorders.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
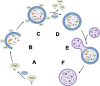
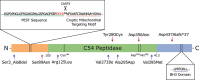
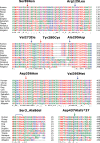
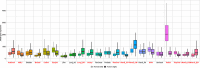
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
