Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide-MXene-Multi-Walled Carbon Nanotubes Electrode
- PMID: 38920565
- PMCID: PMC11201644
- DOI: 10.3390/bios14060261
Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide-MXene-Multi-Walled Carbon Nanotubes Electrode
Abstract
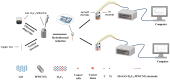
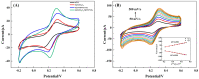
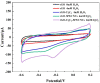
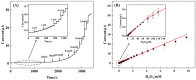
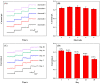
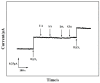
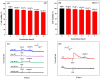
Hydrogen peroxide (H2O2) is a signaling molecule that has the capacity to control a variety of biological processes in organisms. Cancer cells release more H2O2 during abnormal tumor growth. There has been a considerable amount of interest in utilizing H2O2 as a biomarker for the diagnosis of cancer tissue. In this study, an electrochemical sensor for H2O2 was constructed based on 3D reduced graphene oxide (rGO), MXene (Ti3C2), and multi-walled carbon nanotubes (MWCNTs) composite. Three-dimensional (3D) rGO-Ti3C2-MWCNTs sensor showed good linearity for H2O2 in the ranges of 1-60 μM and 60 μM-9.77 mM at a working potential of -0.25 V, with sensitivities of 235.2 µA mM-1 cm-2 and 103.8 µA mM-1 cm-2, respectively, and a detection limit of 0.3 µM (S/N = 3). The sensor exhibited long-term stability, good repeatability, and outstanding immunity to interference. In addition, the modified electrode was employed to detect real-time H2O2 release from cancer cells and cancer tissue ex vivo.
Keywords: H2O2; MWCNTs; MXene; cancer tissue; rGO; real-time detection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Highly sensitive nonenzymatic glucose and H2O2 sensor based on Ni(OH)2/electroreduced graphene oxide--multiwalled carbon nanotube film modified glass carbon electrode.Talanta. 2014 Mar;120:484-90. doi: 10.1016/j.talanta.2013.12.012. Epub 2013 Dec 27. Talanta. 2014. PMID: 24468400
-
Carbon dots-decorated multiwalled carbon nanotubes nanocomposites as a high-performance electrochemical sensor for detection of H2O2 in living cells.Anal Bioanal Chem. 2016 Jul;408(17):4705-14. doi: 10.1007/s00216-016-9554-4. Epub 2016 Apr 23. Anal Bioanal Chem. 2016. PMID: 27108281
-
Real-time electrochemical detection of hydrogen peroxide secretion in live cells by Pt nanoparticles decorated graphene-carbon nanotube hybrid paper electrode.Biosens Bioelectron. 2015 Jun 15;68:358-364. doi: 10.1016/j.bios.2015.01.017. Epub 2015 Jan 8. Biosens Bioelectron. 2015. PMID: 25603401
-
The Simultaneous Detection of Dopamine and Uric Acid In Vivo Based on a 3D Reduced Graphene Oxide-MXene Composite Electrode.Molecules. 2024 Apr 24;29(9):1936. doi: 10.3390/molecules29091936. Molecules. 2024. PMID: 38731427 Free PMC article.
-
Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules.ACS Nano. 2016 Jan 26;10(1):46-80. doi: 10.1021/acsnano.5b05690. Epub 2015 Nov 30. ACS Nano. 2016. PMID: 26579616 Review.
Cited by
-
Functionalized Carbon Nanotubes: Emerging Nanomaterials for Enhanced Cancer Diagnosis and Imaging.Molecules. 2025 May 29;30(11):2364. doi: 10.3390/molecules30112364. Molecules. 2025. PMID: 40509251 Free PMC article. Review.
-
Surface Engineering of MXene and Functional Fullerenols for Cancer Biomarker 'eIF3d'.Langmuir. 2025 Apr 1;41(12):8330-8341. doi: 10.1021/acs.langmuir.5c00157. Epub 2025 Mar 20. Langmuir. 2025. PMID: 40114326 Free PMC article.
-
Recent Advances in MXene-Based Electrochemical Sensors.Biosensors (Basel). 2025 Feb 13;15(2):107. doi: 10.3390/bios15020107. Biosensors (Basel). 2025. PMID: 39997009 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

