Elevated NLRP3 Inflammasome Activation Is Associated with Motor Neuron Degeneration in ALS
- PMID: 38920626
- PMCID: PMC11202041
- DOI: 10.3390/cells13120995
Elevated NLRP3 Inflammasome Activation Is Associated with Motor Neuron Degeneration in ALS
Abstract
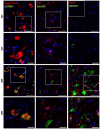
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by motor neuron degeneration in the central nervous system. Recent research has increasingly linked the activation of nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome to ALS pathogenesis. NLRP3 activation triggers Caspase 1 (CASP 1) auto-activation, leading to the cleavage of Gasdermin D (GSDMD) and pore formation on the cellular membrane. This process facilitates cytokine secretion and ultimately results in pyroptotic cell death, highlighting the complex interplay of inflammation and neurodegeneration in ALS. This study aimed to characterize the NLRP3 inflammasome components and their colocalization with cellular markers using the wobbler mouse as an ALS animal model. Firstly, we checked the levels of miR-223-3p because of its association with NLRP3 inflammasome activity. The wobbler mice showed an increased expression of miR-223-3p in the ventral horn, spinal cord, and cerebellum tissues. Next, increased levels of NLRP3, pro-CASP 1, cleaved CASP 1 (c-CASP 1), full-length GSDMD, and cleaved GDSMD revealed NLRP3 inflammasome activation in wobbler spinal cords, but not in the cerebellum. Furthermore, we investigated the colocalization of the aforementioned proteins with neurons, microglia, and astrocyte markers in the spinal cord tissue. Evidently, the wobbler mice displayed microgliosis, astrogliosis, and motor neuron degeneration in this tissue. Additionally, we showed the upregulation of protein levels and the colocalization of NLRP3, c-CASP1, and GSDMD in neurons, as well as in microglia and astrocytes. Overall, this study demonstrated the involvement of NLRP3 inflammasome activation and pyroptotic cell death in the spinal cord tissue of wobbler mice, which could further exacerbate the motor neuron degeneration and neuroinflammation in this ALS mouse model.
Keywords: NLRP3 inflammasome; amyotrophic lateral sclerosis; miR-223-3p; motor neuron degeneration; pyroptotic cell death; wobbler mouse.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Falconer D.S. Wobbler (Wr) Mouse News Lett. 1956;15:23–29.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

